Neural Basis of Touch and Proprioception in Primate Cortex
- PMID: 30215864
- PMCID: PMC6330897
- DOI: 10.1002/cphy.c170033
Neural Basis of Touch and Proprioception in Primate Cortex
Abstract
The sense of proprioception allows us to keep track of our limb posture and movements and the sense of touch provides us with information about objects with which we come into contact. In both senses, mechanoreceptors convert the deformation of tissues-skin, muscles, tendons, ligaments, or joints-into neural signals. Tactile and proprioceptive signals are then relayed by the peripheral nerves to the central nervous system, where they are processed to give rise to percepts of objects and of the state of our body. In this review, we first examine briefly the receptors that mediate touch and proprioception, their associated nerve fibers, and pathways they follow to the cerebral cortex. We then provide an overview of the different cortical areas that process tactile and proprioceptive information. Next, we discuss how various features of objects-their shape, motion, and texture, for example-are encoded in the various cortical fields, and the susceptibility of these neural codes to attention and other forms of higher-order modulation. Finally, we summarize recent efforts to restore the senses of touch and proprioception by electrically stimulating somatosensory cortex. © 2018 American Physiological Society. Compr Physiol 8:1575-1602, 2018.
Copyright © 2018 American Physiological Society. All rights reserved.
Figures
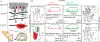
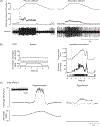
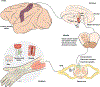
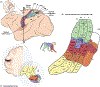







References
-
- Ageranioti-Be´langer SA, Chapman CE. Discharge properties of neurones in the hand area of primary somatosensory cortex in monkeys in relation to the performance of an active tactile discrimination task. II. Area 2 as compared to areas 3b and 1. Exp brain Res 91: 207–228, 1992. - PubMed
-
- Andersen RA, Asanuma C, Essick GK, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296: 65–113, 1990. - PubMed
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

