Dissection of Fragmentation Pathways in Protonated N-Acetylhexosamines
- PMID: 30216047
- PMCID: PMC6490963
- DOI: 10.1021/acs.analchem.8b01963
Dissection of Fragmentation Pathways in Protonated N-Acetylhexosamines
Abstract
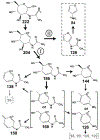
Structural characterization of carbohydrates by mass spectrometry necessitates a detailed understanding of their gas phase behavior, particularly for protonated carbohydrates that can undergo complex structural rearrangements during fragmentation. Here we utilize tandem mass spectrometry, isotopic labeling, gas-phase hydrogen/deuterium exchange, and ion mobility measurements to characterize structures of the various product ions of protonated N-acetylhexosamines. Following the facile loss of the reducing end hydroxyl group, we identify two primary fragmentation pathways. Detailed mapping of each step in the fragmentation pathway provides new insight into the mechanisms that drive collision-induced dissociation of protonated carbohydrates. Several of the smaller fragment ions are mixtures of structural isomers, and the relative distributions of these structures reveals information about the stereochemistry of the precursor molecule.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Varki A, Cummings R, Esko J, Stanley P, Hart GW, Aebi M, Darvill A, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, Essentials of Glycobiology. 3rd ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, 2015. - PubMed
-
- Domon B, Costello CE, A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J 1988, 5 397–409, DOI: 10.1007/bf01049915. - DOI
-
- Richter WJ, Muller DR, Domon B, Tandem mass spectrometry in structural characterization of oligosaccharide residues in glycoconjugates. Methods Enzymol 1990, 193 607–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

