Warsaw breakage syndrome: Further clinical and genetic delineation
- PMID: 30216658
- PMCID: PMC6289708
- DOI: 10.1002/ajmg.a.40482
Warsaw breakage syndrome: Further clinical and genetic delineation
Abstract
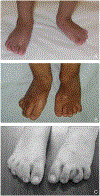
Warsaw breakage syndrome (WBS) is a recently recognized DDX11-related rare cohesinopathy, characterized by severe prenatal and postnatal growth restriction, microcephaly, developmental delay, cochlear anomalies, and sensorineural hearing loss. Only seven cases have been reported in the English literature, and thus the information on the phenotype and genotype of this interesting condition is limited. We provide clinical and molecular information on five additional unrelated patients carrying novel bi-allelic variants in the DDX11 gene, identified via whole exome sequencing. One of the variants was found to be a novel Saudi founder variant. All identified variants were classified as pathogenic or likely pathogenic except for one that was initially classified as a variant of unknown significance (VOUS) (p.Arg378Pro). Functional characterization of this VOUS using heterologous expression of wild type and mutant DDX11 revealed a marked effect on protein stability, thus confirming pathogenicity of this variant. The phenotypic data of the seven WBS reported patients were compared to our patients for further phenotypic delineation. Although all the reported patients had cochlear hypoplasia, one patient also had posterior labyrinthine anomaly. We conclude that while the cardinal clinical features in WBS (microcephaly, growth retardation, and cochlear anomalies) are almost universally present, the breakage phenotype is highly variable and can be absent in some cases. This report further expands the knowledge of the phenotypic and molecular features of WBS.
Keywords: DDX11; Warsaw breakage syndrome; cochlear anomalies; cohesinopathy; exome sequencing; growth restriction; hearing loss; microcephaly.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures






References
-
- Abouelhoda M, Sobahy T, El-Kalioby M, Patel N, Shamseldin H, Monies D, Al-Tassan N, Ramzan K, Imtiaz F, Shaheen R, Alkuraya FS. 2016. Clinical genomics can facilitate countrywide estimation of autosomal recessive disease burden. Genet. Med. Off. J. Am. Coll. Med. Genet 18: 1244–1249. - PubMed
-
- Alfares A, Aloraini T, Subaie LA, Alissa A, Qudsi AA, Alahmad A, Mutairi FA, Alswaid A, Alothaim A, Eyaid W, Albalwi M, Alturki S, Alfadhel M. 2018. Whole-genome sequencing offers additional but limited clinical utility compared with reanalysis of whole-exome sequencing. Genet. Med - PubMed
-
- Bailey C, Fryer AE, Greenslade M. 2015. Warsaw Breakage Syndrome – A further report, emphasising cutaneous findings. Eur. J. Med. Genet 58: 235–237. - PubMed
-
- Capo-Chichi J-M, Bharti SK, Sommers JA, Yammine T, Chouery E, Patry L, Rouleau GA, Samuels ME, Hamdan FF, Michaud JL, Brosh RM Jr, Mégarbane A, Kibar Z. 2013. Identification and Biochemical Characterization of a Novel Mutation in DDX11 Causing Warsaw Breakage Syndrome. Hum. Mutat 34: 103–107. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

