The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies
- PMID: 30217007
- PMCID: PMC6162647
- DOI: 10.3390/cancers10090327
The Role of Signal Transducer and Activator of Transcription 3 (STAT3) and Its Targeted Inhibition in Hematological Malignancies
Abstract
Signal transducer and activator of transcription 3 (STAT3), a member of the STAT protein family, can be phosphorylated by receptor-associated Janus kinases (JAKs) in response to stimulation by cytokines and growth factors. It forms homo- or heterodimers that can translocate to the cell nucleus where they act as transcription activators. Constitutive activation of STAT3 has been found to be associated with initiation and progression of various cancers. It can exert proliferative as well as anti-apoptotic effects. This review focuses on the role of STAT3 in pathogenesis i.e., proliferation, differentiation, migration, and apoptosis of hematological malignancies viz. leukemia, lymphoma and myeloma, and briefly highlights the potential therapeutic approaches developed against STAT3 activation pathway.
Keywords: STAT3; anti-apoptosis; hematological malignancies; proliferation; targeted inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
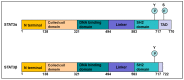
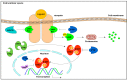

References
-
- Darnell J.E., Jr. The JAK-STAT pathway: Summary of initial studies and recent advances. Recent Prog. Horm. Res. 1996;51:391–403. - PubMed
-
- Siveen K.S., Nguyen A.H., Lee J.H., Li F., Singh S.S., Kumar A.P., Low G., Jha S., Tergaonkar V., Ahn K.S., et al. Negative regulation of signal transducer and activator of transcription-3 signalling cascade by lupeol inhibits growth and induces apoptosis in hepatocellular carcinoma cells. Br. J. Cancer. 2014;111:1327–1337. doi: 10.1038/bjc.2014.422. - DOI - PMC - PubMed
-
- Subramaniam A., Shanmugam M.K., Ong T.H., Li F., Perumal E., Chen L., Vali S., Abbasi T., Kapoor S., Ahn K.S., et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 2013;170:807–821. doi: 10.1111/bph.12302. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

