The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations
- PMID: 30217041
- PMCID: PMC6162705
- DOI: 10.3390/jcm7090280
The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations
Abstract
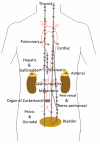
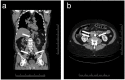
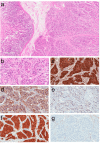
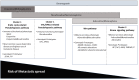
Paragangliomas are neuroendocrine neoplasms, derived from paraganglia of the sympathetic and parasympathetic nervous systems. They are most commonly identified in the head and neck, being most frequent in the carotid body, followed by jugulotympanic paraganglia, vagal nerve and ganglion nodosum, as well as laryngeal paraganglia. Abdominal sites include the well-known urinary bladder tumors that originate in the Organ of Zuckerkandl. However, other unusual sites of origin include peri-adrenal, para-aortic, inter-aortocaval, and paracaval retroperitoneal sites, as well as tumors in organs where they may not be expected in the differential diagnosis of neuroendocrine neoplasms, such as thyroid, parathyroid, pituitary, gut, pancreas, liver, mesentery, lung, heart and mediastinum. The distinction of these lesions from epithelial neuroendocrine neoplasms is critical for several reasons. Firstly, the determination of clinical and biochemical features is different from that used for epithelial neuroendocrine tumors. Secondly, the genetic implications are different, since paragangliomas/pheochromocytomas have the highest rate of germline susceptibility at almost 40%. Finally, the characterization of metastatic disease is unique in these highly syndromic lesions. In this review, we summarize updated concepts by outlining the spectrum of anatomic locations of paragangliomas, the importance of morphology in establishing the correct diagnosis, the clinical implications for management, and the impact of genetics on the distinction between multifocal primary tumors compared with malignant disease.
Keywords: SDHB; catecholamines; genetic susceptibility; metastatic paraganglioma; paraganglioma; pheochromocytoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rindi G., Klimstra D.S., Abedi-Ardekani B., Asa S.L., Bosman F.T., Brambilla E., Busam K.J., de Krijger R.R., Dietel M., El-Naggar A.K., et al. A common classification framework for neuroendocrine neoplasms: An international Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018 doi: 10.1038/s41379-018-0110-y. - DOI - PMC - PubMed
-
- Lack E.E. Atlas of Tumor Pathology. American Registry of Pathology Press; Washington, DC, USA: 2007. Tumors of the Adrenal Gland and Extraadrenal Paraganglia. Armed Forces Institute of Pathology. (Series 4, Faccicle 8).
-
- Tischler A.S. The adrenal medulla and extra-adrenal paraganglia. In: Kovacs K., Asa S.L., editors. Functional Endocrine Pathology. Blackwell Science; Hoboken, NJ, USA: 1998. pp. 550–595.
-
- Oudijk L., de Krijger R.R., Pacak K., Tischler A.S. Adrenal medulla and extra-adrenal paraganglia. In: Mete O., Asa S.L., editors. Endocrine Pathology. Cambridge University Press; Cambridge, UK: 2016. pp. 628–676.
-
- Hayashi T., Mete O. Head and neck paragangliomas: What does the pathologist need to know? Diagn. Histopathol. 2014;20:316–325. doi: 10.1016/j.mpdhp.2014.06.002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

