Variability of Botulinum Toxins: Challenges and Opportunities for the Future
- PMID: 30217070
- PMCID: PMC6162648
- DOI: 10.3390/toxins10090374
Variability of Botulinum Toxins: Challenges and Opportunities for the Future
Abstract
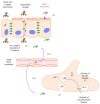
Botulinum neurotoxins (BoNTs) are the most potent known toxins, and are therefore classified as extremely harmful biological weapons. However, BoNTs are therapeutic drugs that are widely used and have an increasing number of applications. BoNTs show a high diversity and are divided into multiple types and subtypes. Better understanding of the activity at the molecular and clinical levels of the natural BoNT variants as well as the development of BoNT-based chimeric molecules opens the door to novel medical applications such as silencing the sensory neurons at targeted areas and dermal restoration. This short review is focused on BoNTs' variability and the opportunities or challenges posed for future clinical applications.
Keywords: BoNT variants; botulinum neurotoxins (BoNTs); subtypes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Challenges in searching for therapeutics against Botulinum Neurotoxins.Expert Opin Drug Discov. 2017 May;12(5):497-510. doi: 10.1080/17460441.2017.1303476. Epub 2017 Mar 17. Expert Opin Drug Discov. 2017. PMID: 28271909 Review.
-
Critical Analysis of Neuronal Cell and the Mouse Bioassay for Detection of Botulinum Neurotoxins.Toxins (Basel). 2019 Dec 7;11(12):713. doi: 10.3390/toxins11120713. Toxins (Basel). 2019. PMID: 31817843 Free PMC article. Review.
-
Comparison of extracellular and intracellular potency of botulinum neurotoxins.Infect Immun. 2006 Oct;74(10):5617-24. doi: 10.1128/IAI.00552-06. Infect Immun. 2006. PMID: 16988237 Free PMC article.
-
Emerging Opportunities in Human Pluripotent Stem-Cells Based Assays to Explore the Diversity of Botulinum Neurotoxins as Future Therapeutics.Int J Mol Sci. 2021 Jul 14;22(14):7524. doi: 10.3390/ijms22147524. Int J Mol Sci. 2021. PMID: 34299143 Free PMC article. Review.
-
P19 embryonal carcinoma cells exhibit high sensitivity to botulinum type C and D/C mosaic neurotoxins.Microbiol Immunol. 2012 Oct;56(10):664-72. doi: 10.1111/j.1348-0421.2012.00490.x. Microbiol Immunol. 2012. PMID: 22738015
Cited by
-
SNARE protein SNAP25 regulates the chloride-transporter KCC2 in neurons.iScience. 2024 Oct 11;27(11):111156. doi: 10.1016/j.isci.2024.111156. eCollection 2024 Nov 15. iScience. 2024. PMID: 39507243 Free PMC article.
-
Embracing the Versatility of Botulinum Neurotoxins in Conventional and New Therapeutic Applications.Toxins (Basel). 2024 Jun 4;16(6):261. doi: 10.3390/toxins16060261. Toxins (Basel). 2024. PMID: 38922155 Free PMC article. Review.
-
Biosecurity Threat Posed by Botulinum Toxin.Toxins (Basel). 2019 Nov 20;11(12):681. doi: 10.3390/toxins11120681. Toxins (Basel). 2019. PMID: 31757074 Free PMC article. Review.
-
Transdermal Delivery of Botulinum Neurotoxin A: A Novel Formulation with Therapeutic Potential.Pharmaceutics. 2025 Jan 22;17(2):146. doi: 10.3390/pharmaceutics17020146. Pharmaceutics. 2025. PMID: 40006513 Free PMC article.
-
An outbreak of type C botulism in free-ranging Southern lapwing (Vanellus chilensis).Vet Res Commun. 2024 Apr;48(2):1239-1243. doi: 10.1007/s11259-023-10264-1. Epub 2023 Nov 27. Vet Res Commun. 2024. PMID: 38008781
References
-
- Brook I. Clostridium Botulinum: A Spore Forming Organism and a Challenge to Food Safety. Nova Science Publishers; Hauppauge, NJ, USA: 2012. Botulism symptoms, incubation period and complications, diagnosis and management. Advances in Food Safety and Food Microbiology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

