Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials
- PMID: 30217097
- PMCID: PMC6164106
- DOI: 10.3390/ma11091716
Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials
Abstract
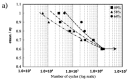
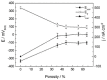
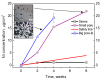
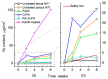
Porous shape memory alloys (SMAs), including NiTi and Ni-free Ti-based alloys, are unusual materials for hard-tissue replacements because of their unique superelasticity (SE), good biocompatibility, and low elastic modulus. However, the Ni ion releasing for porous NiTi SMAs in physiological conditions and relatively low SE for porous Ni-free SMAs have delayed their clinic applications as implantable materials. The present article reviews recent research progresses on porous NiTi and Ni-free SMAs for hard-tissue replacements, focusing on two specific topics: (i) synthesis of porous SMAs with optimal porous structure, microstructure, mechanical, and biological properties; and, (ii) surface modifications that are designed to create bio-inert or bio-active surfaces with low Ni releasing and high biocompatibility for porous NiTi SMAs. With the advances of preparation technique, the porous SMAs can be tailored to satisfied porous structure with porosity ranging from 30% to 85% and different pore sizes. In addition, they can exhibit an elastic modulus of 0.4⁻15 GPa and SE of more than 2.5%, as well as good cell and tissue biocompatibility. As a result, porous SMAs had already been used in maxillofacial repairing, teeth root replacement, and cervical and lumbar vertebral implantation. Based on current research progresses, possible future directions are discussed for "property-pore structure" relationship and surface modification investigations, which could lead to optimized porous biomedical SMAs. We believe that porous SMAs with optimal porous structure and a bioactive surface layer are the most competitive candidate for short-term and long-term hard-tissue replacement materials.
Keywords: NiTi; biocompatibility; porous material; shape memory alloy; surface modification; β type Ni-free Ti alloy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



























References
-
- World Population Prospects 2017. Population Division, DESA, United Nations; New York, NY, USA: 2017.
-
- Wise D.L. Biomaterials Engineering and Devices. Humana Press; Berlin, Germany: 2000. pp. 205–319.
-
- Park J.B., Bronzino J.D. Biomaterials: Principles and Applications. CRC Press; Boca Rator, FL, USA: 2003. pp. 1–241.
-
- Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–A review. Prog. Mater. Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. - DOI
Publication types
Grants and funding
- 51571090/National Natural Science Foundation of China
- 51621001/Innovative Research Groups of the National Natural Science Foundation of China
- 2017B030308001/Training Program of Major Basic Research Project of Provincial Natural Science Foundation of Guangdong
- 2017ZD009/the Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

