Ambiguous Effects of Autophagy Activation Following Hypoperfusion/Ischemia
- PMID: 30217100
- PMCID: PMC6163197
- DOI: 10.3390/ijms19092756
Ambiguous Effects of Autophagy Activation Following Hypoperfusion/Ischemia
Abstract
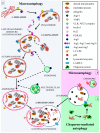
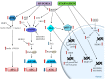
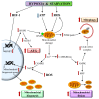
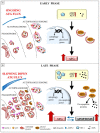
Autophagy primarily works to counteract nutrient deprivation that is strongly engaged during starvation and hypoxia, which happens in hypoperfusion. Nonetheless, autophagy is slightly active even in baseline conditions, when it is useful to remove aged proteins and organelles. This is critical when the mitochondria and/or proteins are damaged by toxic stimuli. In the present review, we discuss to that extent the recruitment of autophagy is beneficial in counteracting brain hypoperfusion or, vice-versa, its overactivity may per se be detrimental for cell survival. While analyzing these opposite effects, it turns out that the autophagy activity is likely not to be simply good or bad for cell survival, but its role varies depending on the timing and amount of autophagy activation. This calls for the need for an appropriate autophagy tuning to guarantee a beneficial effect on cell survival. Therefore, the present article draws a theoretical pattern of autophagy activation, which is hypothesized to define the appropriate timing and intensity, which should mirrors the duration and severity of brain hypoperfusion. The need for a fine tuning of the autophagy activation may explain why confounding outcomes occur when autophagy is studied using a rather simplistic approach.
Keywords: autophagy; brain ischemia; cerebral blood flow; hypoxia; mitophagy; neurodegeneration; starvation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage.Neuropharmacology. 2014 Nov;86:103-15. doi: 10.1016/j.neuropharm.2014.07.002. Epub 2014 Jul 10. Neuropharmacology. 2014. PMID: 25018043
-
Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo.CNS Neurosci Ther. 2019 Sep;25(9):976-986. doi: 10.1111/cns.13136. Epub 2019 Apr 11. CNS Neurosci Ther. 2019. PMID: 30972969 Free PMC article.
-
Modulation of Autophagy Through Regulation of 5'-AMP-Activated Protein Kinase Affects Mitophagy and Mitochondrial Function in Primary Human Trophoblasts.Reprod Sci. 2021 Aug;28(8):2314-2322. doi: 10.1007/s43032-021-00495-5. Epub 2021 Feb 22. Reprod Sci. 2021. PMID: 33619701
-
Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay.Biochim Biophys Acta. 2015 Nov;1847(11):1412-23. doi: 10.1016/j.bbabio.2015.04.010. Epub 2015 Apr 24. Biochim Biophys Acta. 2015. PMID: 25917894 Review.
-
Review: autophagy and neurodegeneration: survival at a cost?Neuropathol Appl Neurobiol. 2010 Apr;36(2):125-32. doi: 10.1111/j.1365-2990.2010.01062.x. Epub 2010 Feb 19. Neuropathol Appl Neurobiol. 2010. PMID: 20202120 Free PMC article. Review.
Cited by
-
mTOR-Related Cell-Clearing Systems in Epileptic Seizures, an Update.Int J Mol Sci. 2020 Feb 28;21(5):1642. doi: 10.3390/ijms21051642. Int J Mol Sci. 2020. PMID: 32121250 Free PMC article. Review.
-
Mild hypothermia pretreatment improves hepatic ischemia-reperfusion injury: A systematic review and meta-analysis of animal experiments.PLoS One. 2024 Jul 2;19(7):e0305213. doi: 10.1371/journal.pone.0305213. eCollection 2024. PLoS One. 2024. PMID: 38954712 Free PMC article.
-
Autophagy is a pro-survival adaptive response to heat shock in bovine cumulus-oocyte complexes.Sci Rep. 2020 Aug 13;10(1):13711. doi: 10.1038/s41598-020-69939-3. Sci Rep. 2020. PMID: 32792582 Free PMC article.
-
Transcriptional block of AMPK-induced autophagy promotes glutamate excitotoxicity in nutrient-deprived SH-SY5Y neuroblastoma cells.Cell Mol Life Sci. 2020 Sep;77(17):3383-3399. doi: 10.1007/s00018-019-03356-2. Epub 2019 Nov 12. Cell Mol Life Sci. 2020. PMID: 31720741 Free PMC article.
-
Autophagy in glaucoma pathogenesis: Therapeutic potential and future perspectives.Front Cell Dev Biol. 2022 Dec 14;10:1068213. doi: 10.3389/fcell.2022.1068213. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36589756 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

