Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort
- PMID: 30217169
- PMCID: PMC6137907
- DOI: 10.1186/s12879-018-3367-3
Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort
Abstract
Background: As direct acting antiviral (DAA) therapy is progressively rolled out for patients with hepatitis C virus (HCV) infection, careful scrutiny of HCV epidemiology, diagnostic testing, and access to care is crucial to underpin improvements in delivery of treatment, with the ultimate goal of elimination.
Methods: We retrospectively studied microbiology records from a large UK teaching hospital in order to compare the performance of HCV screening and diagnostic tests (antibody, antigen and HCV RNA detection). Having described a local cohort of adults with active HCV infection, we investigated the proportion who attended hospital appointments, were prescribed direct acting antiviral (DAA) therapy, and cleared HCV RNA following treatment.
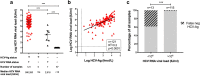
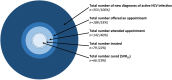
Results: Over a total time period of 33 months between 2013 and 2016, we tested 38,509 individuals for HCV infection and confirmed a new diagnosis of active HCV infection (HCV-Ag + and/or HCV RNA+) in 353 (positive rate 0.9%). Our in-house HCV-Ab screening test had a positive predictive value of 87% compared to repeat HCV-Ab testing in a reference laboratory, highlighting the potential for false positives to arise using this test. HCV-Ag had 100% positive predictive value compared to detection of HCV RNA. There was a strong correlation between quantitative HCV-Ag and HCV RNA viral load (p < 0.0001). Among the cases of infection, genotype-1 and genotype-3 predominated, the median age was 37 years, 84% were male, and 36% were in prison. Hepatology review was provided in 39%, and 22% received treatment. Among those who received DAA therapy with 12 weeks of follow-up, 93% achieved a sustained virologic response (SVR12).
Conclusions: HCV-Ag performs well as a diagnostic test compared to PCR for HCV RNA. Active HCV infection is over-represented among men and in the prison population. DAA therapy is successful in those who receive it, but a minority of patients with a diagnosis of HCV infection access clinical care. Enhanced efforts are required to provide linkage to clinical care within high risk populations.
Keywords: Antibody; Antigen; Cure; DAA; Diagnosis; Epidemiology; Ethnicity; Genotype; HCV; Prison; Screening; Sustainable development goals; Treatment.
Conflict of interest statement
Authors’ information
EB is the lead for the UK STOP-HCV program. PCM is a Wellcome Trust Clinical Research Fellow investigating chronic viral hepatitis infection.
Ethics approval and consent to participate
No specific ethics approval was required for this study as it was undertaken as a quality improvement study using anonymised data from within a clinical microbiology laboratory and hepatology service.
Consent for publication
Not applicable.
Competing interests
MA has received research funding from Gilead. PCM is a member of BMC Infectious Diseases Editorial Board (assistant editor).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

