Effect of interferon beta-1a subcutaneously three times weekly on clinical and radiological measures and no evidence of disease activity status in patients with relapsing-remitting multiple sclerosis at year 1
- PMID: 30217172
- PMCID: PMC6137887
- DOI: 10.1186/s12883-018-1145-x
Effect of interferon beta-1a subcutaneously three times weekly on clinical and radiological measures and no evidence of disease activity status in patients with relapsing-remitting multiple sclerosis at year 1
Abstract
Background: In the PRISMS study, interferon beta-1a subcutaneously (IFN β-1a SC) reduced clinical and radiological disease burden at 2 years in patients with relapsing-remitting multiple sclerosis. The study aimed to characterize efficacy of IFN β-1a SC 44 μg and 22 μg three times weekly (tiw) at Year 1.
Methods: Exploratory endpoints included annualized relapse rate (ARR), 3-month confirmed disability progression (1-point Expanded Disability Status Scale increase if baseline was < 6.0 [0.5-point if baseline was ≥6.0]), active T2 lesions, and no evidence of disease activity (NEDA; defined as no relapses [subanalyzed by relapse severity], 3-month confirmed progression, or active T2 lesions). Effect of IFN β-1a SC in prespecified patient subgroups was also assessed.
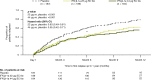
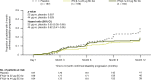
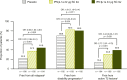
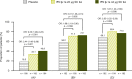
Results: Patients were randomized to IFN β-1a 22 μg (n = 189), 44 μg (n = 184), or placebo (n = 187). At 1 year, IFN β-1a SC tiw reduced ARR (p < 0.001), risk of disability progression (p ≤ 0.029), and mean number of active T2 lesions per patients per scan (p < 0.001) versus placebo. Clinical and radiological benefits were seen as early as Month 2 and 3. Outcomes in subgroups were consistent with those in the overall population. More patients treated with IFN β-1a SC tiw achieved NEDA status, versus placebo, regardless of relapse severity (p ≤ 0.006).
Conclusion: Clinical, radiological, and NEDA outcomes at Year 1 were consistent with Year 2 results. Treatment efficacy was consistent in pre-specified patient subgroups.
Keywords: Clinical trials; Disability progression; Interferon-beta subcutaneously; MRI; No evidence of disease activity; Relapsing–remitting multiple sclerosis.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from appropriate Institutional Ethics Committees/Institutional Review Boards: Royal Melbourne Hospital, Melbourne, Victoria, Australia; Central Sydney Area Health Service, Camperdown, Australia; University of Western Ontario, London, Ontario, Canada; Ottawa General Hospital, Ottawa, Ontario, Canada; University of British Columbia, Vancouver, British Columbia, Canada; Lund University Hospital, Lund, Sweden; Independent Review Board, Amsterdam, Netherlands; Newcastle & North Tyneside Health Authorities, Newcastle upon Tyne, UK; University Hospital, Nottingham, UK; St. Thomas’s Hospital, London, UK; Central Oxford Research Ethics Committee, Headington, Oxford, UK; UZ Leuven, Leuven, Belgium; Limburgs Universitair Centrum, Diepenbeek, Belgium; Université catholique de Louvain, Louvain-la-Neuve, Belgium; Helsinki University Hospitals, Helsinki, Finland; Turku University Central Hospital, Turku, Finland; Würzburg University, Würzburg, Germany; Kantonsspital Basel, Basel, Switzerland; Academisch Ziekenhuis Rotterdam, Rotterdam, Netherlands; St George’s Hospital, London, UK; and Hôpitaux Universitaires de Genève, Geneva, Switzerland.. All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
A Traboulsee has acted as a consultant for Biogen, Genzyme, Roche, and Teva, and is Principal Investigator on clinical trials for Biogen, Chugai, Genzyme, and Roche.
D Li is the Director of the UBC MS/MRI Research Group, which has been contracted to perform central analysis of MRI scans for therapeutic trials with Genzyme, Hoffmann-La Roche, Merck Serono, Nuron, Perspectives, and Sanofi-Aventis. He has acted as a consultant to Vertex Pharmaceuticals; has served on scientific advisory boards for Novartis, Nuron, and Roche; has served on a data and safety advisory board for Opexa; and has received research funding from the Canadian Institute of Health Research and Multiple Sclerosis Society of Canada.
M Cascione has received funding/honoraria for research, consultation, and speakers bureau participation from Acorda, Bayer HealthCare, Biogen, EMD Serono, Genentech, Genzyme/Sanofi, Novartis, Pfizer, Roche, and Teva Pharmaceuticals.
J Fang was an employee of EMD Serono, Inc., Rockland, MA, USA (a business of Merck KGaA, Darmstadt, Germany) at the time of writing.
F Dangond is an employee of EMD Serono, Inc., Billerica, MA, USA (a business of Merck KGaA, Darmstadt, Germany).
A Miller has received research support from Biogen, Genzyme/Sanofi, Mallinckrodt (Questcor), Novartis, and Roche/Genentech. He has acted as a consultant for Accordant Health Services (Caremark), Acorda Therapeutics, Alkermes, Biogen, EMD Serono, Sanofi Genzyme, GlaxoSmithKline, Mallinckrodt (Questcor), Novartis, and Roche/Genentech. He has served on the speakers bureau for Biogen, Genentech, and Sanofi Genzyme for unbranded disease awareness programs only.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

