Anticholinergic burden and comorbidities in patients attending treatment with trospium chloride for overactive bladder in a real-life setting: results of a prospective non-interventional study
- PMID: 30217174
- PMCID: PMC6137886
- DOI: 10.1186/s12894-018-0394-8
Anticholinergic burden and comorbidities in patients attending treatment with trospium chloride for overactive bladder in a real-life setting: results of a prospective non-interventional study
Abstract
Background: Elderly people are representative for the patients most likely to be treated with anticholinergics for overactive bladder (OAB). They often receive further drugs with anticholinergic properties for concomitant conditions. This increases the risk for side effects, including central nervous system disorders. Data on comorbidities and baseline anticholinergic burden of OAB patients seen in urological practice is scarce. Therefore, we included an epidemiological survey on these issues in our study which assessed the effectiveness and tolerability of trospium chloride (TC) in established dosages under routine conditions.
Methods: Outpatients (≥ 65 years of age), for whom treatment with TC was indicated, were eligible to participate in this non-interventional, prospective study performed in 162 urological practices in Germany. Epidemiological questions were evaluated by the Anticholinergic Burden (ACB) scale and the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) at baseline. Efficacy was assessed by changes in symptom-related variables of OAB after treatment. Dosage regimen, duration of treatment, adverse events, withdrawals, and ease of subdivision of the prescribed SNAP-TAB tablet were documented. Patients and physicians rated efficacy and tolerability of treatment. Statistics were descriptive.
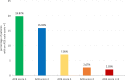
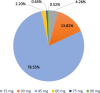
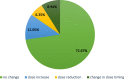
Results: Four hundred fourty-five out of 986 (47.54%) patients in the epidemiological population had a baseline ACB scale score > 0, 100 (24.72%) of whom a score ≥ 3. The median CIRS-G comorbidity index score for all patients was 5. 78.55% (608/774) of patients in the efficacy population received a daily dose of 45 mg TC. 60.03% (365/608) of them took this dose by dividing the SNAP-TAB tablet in three equal parts. Before-after-comparisons of the core symptoms of OAB showed clear improvements. An influence of the dosage scheme (1 × 45 mg TC/d vs 3 × 15 mg TC/d) on clinical outcome could not be observed. Most urologists and patients rated TC treatment as effective and well tolerated. 44 (4.37%) out of 1007 patients in the safety collective ended their treatment prematurely, while 75 patients (7.45%) experienced adverse events.
Conclusions: Anticholinergic burden and comorbidities in elderly OAB patients are frequent. The acceptance of the SNAP-TAB tablet, which facilitates flexible dosing with TC, was high, which is supportive in ensuring adherence in therapy.
Trial registration: This non-interventional study was registered on October 29, 2014 with the number DRKS00007109 at the German Register of Clinical Studies (DRKS).
Keywords: Anticholinergic burden; CIRS-G; Comorbidity index; Elderly patients; Non-interventional study; Overactive bladder; Trospium chloride.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the German Drug Law, with the joint recommendations of the Federal Institute for Drugs and Medical Devices (BfArM) and the Paul-Ehrlich-Institute relating to the conduct of non-interventional trials [37], and with the recommendations for assuring Good Epidemiological Practice [38]. Written informed consent regarding data protection was obtained from all patients before being included in the study. The Ethics Committee of the Medical Chamber Westphalia-Lippe and the Westphalian Wilhelm University Münster, Germany, approved the study protocol. This decision was provided to each urologist within the regulatory jurisdiction of the Ethics Committee. No further Ethics Committee was involved as ethics approval in general was not mandatory and all participating urologists were satisfied with the vote of the Ethics Committee of the Medical Chamber Westphalia-Lippe and Westphalian Wilhelm University Münster.This NIS was registered with the number DRKS00007109 at the German Register of Clinical Studies (DRKS) on October 29, 2014.
Consent for publication
Not applicable.
Competing interests
Alexander Ivchenko received cost reimbursement of travel expenses by Dr. R. Pfleger GmbH. Rolf-Hasso Bödeker is paid-consultant (statistical planning and analysing) to Dr. R. Pfleger GmbH, Andreas Wiedemann is a consultant of Dr. R. Pfleger GmbH, Claudia Neumeister is employee (Senior Project Manager Clinical Research) of Dr. R. Pfleger GmbH.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effect of trospium chloride therapy on intraocular pressure and tear secretion in overactive bladder patients.Cutan Ocul Toxicol. 2017 Dec;36(4):331-335. doi: 10.1080/15569527.2017.1289219. Epub 2017 May 4. Cutan Ocul Toxicol. 2017. PMID: 28468509 Clinical Trial.
-
[Trospium chloride once daily for overactive bladder syndrome: results of a multicenter observational study].Urologe A. 2013 Jan;52(1):65-70. doi: 10.1007/s00120-012-2989-0. Urologe A. 2013. PMID: 23052979 Clinical Trial. German.
-
Study of the population pharmacokinetic characteristics of once-daily trospium chloride 60 mg extended-release capsules in patients with overactive bladder and in healthy subjects.Clin Drug Investig. 2013 Feb;33(2):133-41. doi: 10.1007/s40261-012-0039-8. Clin Drug Investig. 2013. PMID: 23203138
-
Trospium chloride treatment of overactive bladder.Ann Pharmacother. 2009 Feb;43(2):283-95. doi: 10.1345/aph.1L160. Epub 2009 Feb 3. Ann Pharmacother. 2009. PMID: 19193592 Review.
-
Cognitive Effects of Anticholinergic Load in Women with Overactive Bladder.Clin Interv Aging. 2020 Aug 25;15:1493-1503. doi: 10.2147/CIA.S252852. eCollection 2020. Clin Interv Aging. 2020. PMID: 32921995 Free PMC article. Review.
Cited by
-
Validating 7-items Overactive Bladder Symptom Score (OABSS) through Arabic linguistic version.Sci Rep. 2021 Jan 12;11(1):661. doi: 10.1038/s41598-020-79974-9. Sci Rep. 2021. PMID: 33436744 Free PMC article.
-
Development and validation of models predicting treatment patterns in women with urinary urgency and/or urgency incontinence: A Symptoms of Lower Urinary Tract Dysfunction Research Network observational cohort study.Neurourol Urodyn. 2023 Aug;42(6):1214-1226. doi: 10.1002/nau.25222. Epub 2023 Jun 3. Neurourol Urodyn. 2023. PMID: 37269483 Free PMC article.
-
Overactive Bladder Prescribing Considerations: The Role of Polypharmacy, Anticholinergic Burden, and CYP2D6 Drug‒Drug Interactions.Clin Drug Investig. 2021 Apr;41(4):293-302. doi: 10.1007/s40261-021-01020-x. Epub 2021 Mar 12. Clin Drug Investig. 2021. PMID: 33713027 Free PMC article. Review.
-
Treatment patterns in women with urinary urgency and/or urgency urinary incontinence in the symptoms of Lower Urinary Tract Dysfunction Research Network Observational Cohort Study.Neurourol Urodyn. 2023 Jan;42(1):194-204. doi: 10.1002/nau.25067. Epub 2022 Oct 23. Neurourol Urodyn. 2023. PMID: 36579974 Free PMC article.
-
Anticholinergic burden: First comprehensive analysis using claims data shows large variation by age and sex.PLoS One. 2021 Jun 30;16(6):e0253336. doi: 10.1371/journal.pone.0253336. eCollection 2021. PLoS One. 2021. PMID: 34191827 Free PMC article.
References
-
- Zellner M, Madersbacher H, Palmtag H, et al, and the P195 Study Group. Trospium chloride and oxybutynin hydrochloride in a German study of adults with urinary urge incontinence: results of a 12-week, multicenter, randomized, double-blind, parallel-group, flexible-dose noninferiority trial. Clin Ther 2009; 31: 2519–2539. - PubMed
-
- Ulshöfer B, Bihr AM, Bödeker RH, et al. Randomised, double-blind, placebo-controlled study on the efficacy and tolerance of trospium chloride in patients with motor urge incontinence. Clin Drug Invest. 2001;21(8):563–569. doi: 10.2165/00044011-200121080-00005. - DOI
-
- Alloussi S, Laval KU, Eckert R, et al. Trospium chloride (Spasmo-lyt®) in patients with motor urge syndrome (detrusor instability): a double-blind, randomised, multicentre, placebo-controlled study. J. Clin Res. 1998;1:439–451.
-
- Jünemann KP, Füsgen I, Svetlana T. Trospium chloride 40 mg – a placebo-controlled, randomised, double-blind clinical trial on the efficacy and tolerability for 3 weeks in patients with urge-syndrome. Eur Urol. 2000;37:84.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

