Evaluation of cell-surface displayed synthetic consensus dengue EDIII cells as a potent oral vaccine candidate
- PMID: 30217208
- PMCID: PMC6138890
- DOI: 10.1186/s12934-018-0994-8
Evaluation of cell-surface displayed synthetic consensus dengue EDIII cells as a potent oral vaccine candidate
Abstract
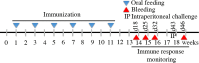
Background: Dengue is a rapidly spreading mosquito borne tropical viral disease affecting hundreds of millions of people across the globe annually. The dengue virus (DENV) includes four genetically distinct serotypes that cause serious life-threatening infections, including dengue hemorrhagic fever/dengue shock syndrome. Dengue vaccine development is complicated by the possibility of vaccine-enhanced severe dengue disease due to antibody-dependent enhancement by pre-existing cross-reactivity, as well as homotypic antibodies. Thus, the development of an efficacious dengue vaccine conferring simultaneous and durable immunity to each of the four DENV serotypes has not yet been developed despite years of research. For mass immunization in deeply affected resource-limited countries, oral vaccination is considered more beneficial than conventional approaches. Therefore, in a continuing effort towards designing economical and potent vaccine candidates, the current study applied yeast surface display technology to develop an oral dengue vaccine candidate using whole recombinant yeast cells displaying the recombinant fusion protein of M cell targeting ligand Co1 fused to the synthetic consensus dengue envelope domain III (scEDIII). Female Balb/c mice were orally fed with recombinant yeast cells and immunogenicity in terms of systemic and mucosal immune responses was monitored.
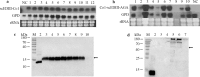
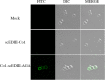
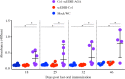
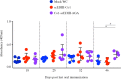
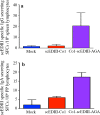
Results: Immunofluorescence microscopy with dengue specific antibody and fluorescein isothiocyanate-conjugated anti-mouse IgG antibody clearly showed that recombinant protein Co1-scEDIII-AGA was localized on the cell surface of the respective clones in comparison with scEDIII-Co1 and Mock cells with no fluorescence. Oral dosage applications of surface displayed Co1-scEDIII-AGA stimulated a systemic humoral immune response in the form of dengue-specific serum IgG, as well as a mucosal immune response in the form of secretory immunoglobulin A (sIgA). Antigen-specific B cell responses in isolated lymphoid cells from the spleen and Peyer's patches further supported an elevated mucosal immune response. In addition, surface displayed Co1-scEDIII-AGA feeding elicited strong immune responses in comparison with scEDIII-Co1 and Mock following intraperitoneal booster with purified scEDIII antigen.
Conclusions: Surface displayed preparations of Co1-scEDIII-AGA induced strong immunogenicity compared with non-displayed scEDIII-Co1. Prior studies have supported the neutralization potential of scEDIII constructs against all four serotypes. Thus, the oral administration of genetically engineered yeast whole cells displaying biologically active Co1-scEDIII fusion protein without any further processing shows prospective as a potent oral vaccine candidate against dengue viral infection.
Keywords: Dengue; Mucosal immunity; Oral vaccine; Saccharomyces cerevisiae; Surface display; scEDIII.
Figures

Similar articles
-
Comparative immunogenicity of preparations of yeast-derived dengue oral vaccine candidate.Microb Cell Fact. 2018 Feb 16;17(1):24. doi: 10.1186/s12934-018-0876-0. Microb Cell Fact. 2018. PMID: 29452594 Free PMC article.
-
Expression and characterization of an M cell-specific ligand-fused dengue virus tetravalent epitope using Saccharomyces cerevisiae.J Biosci Bioeng. 2015 Jan;119(1):19-27. doi: 10.1016/j.jbiosc.2014.06.005. Epub 2014 Jul 12. J Biosci Bioeng. 2015. PMID: 25027708
-
Expression and purification of an immunogenic dengue virus epitope using a synthetic consensus sequence of envelope domain III and Saccharomyces cerevisiae.Protein Expr Purif. 2013 Apr;88(2):235-42. doi: 10.1016/j.pep.2013.01.009. Epub 2013 Jan 31. Protein Expr Purif. 2013. PMID: 23376461
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
Cited by
-
Improving expression and assembly of difficult-to-express heterologous proteins in Saccharomyces cerevisiae by culturing at a sub-physiological temperature.Microb Cell Fact. 2023 Mar 23;22(1):55. doi: 10.1186/s12934-023-02065-7. Microb Cell Fact. 2023. PMID: 36959657 Free PMC article.
-
Yeast oral vaccines against infectious diseases.Front Microbiol. 2023 Apr 17;14:1150412. doi: 10.3389/fmicb.2023.1150412. eCollection 2023. Front Microbiol. 2023. PMID: 37138614 Free PMC article. Review.
-
Mucosal Vaccination: A Promising Alternative Against Flaviviruses.Front Cell Infect Microbiol. 2022 Jun 15;12:887729. doi: 10.3389/fcimb.2022.887729. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782117 Free PMC article. Review.
-
Oral Immunization with Recombinant Saccharomyces cerevisiae Expressing Viral Capsid Protein 2 of Infectious Bursal Disease Virus Induces Unique Specific Antibodies and Protective Immunity.Vaccines (Basel). 2023 Dec 14;11(12):1849. doi: 10.3390/vaccines11121849. Vaccines (Basel). 2023. PMID: 38140252 Free PMC article.
-
A multiple-target mRNA-LNP vaccine induces protective immunity against experimental multi-serotype DENV in mice.Virol Sin. 2022 Oct;37(5):746-757. doi: 10.1016/j.virs.2022.07.003. Epub 2022 Jul 12. Virol Sin. 2022. PMID: 35835315 Free PMC article.
References
-
- WHO. Dengue: guidelines for diagnosis, treatment, prevention, and control. Spec Program Res Train Trop Dis. 2009;409:147. WHO reference number: WHO/HTM/NTD/DEN/2009.1.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

