Clonal analysis reveals laminar fate multipotency and daughter cell apoptosis of mouse cortical intermediate progenitors
- PMID: 30217810
- PMCID: PMC6141770
- DOI: 10.1242/dev.164335
Clonal analysis reveals laminar fate multipotency and daughter cell apoptosis of mouse cortical intermediate progenitors
Abstract
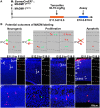
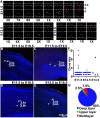
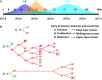
In developing cerebral cortex, most pyramidal-projection neurons are produced by intermediate progenitors (IPs), derived in turn from radial glial progenitors. Although IPs produce neurons for all cortical layers, it is unknown whether individual IPs produce multiple or single laminar fates, and the potential of IPs for extended proliferation remains uncertain. Previously, we found that, at the population level, early IPs (present during lower-layer neurogenesis) produce lower- and upper-layer neurons, whereas late IPs produce upper-layer neurons only. Here, we employed mosaic analysis with double markers (MADM) in mice to sparsely label early IP clones. Most early IPs produced 1-2 neurons for deep layers only. Less frequently, early IPs produced larger clones (up to 12 neurons) spanning lower and upper layers, or upper layers only. The majority of IP-derived clones (∼66%) were associated with asymmetric cell death after the first division. These data demonstrate that laminar fate is not predetermined, at least in some IPs. Rather, the heterogeneous sizes and laminar fates of early IP clones are correlated with cell division/death/differentiation choices and neuron birthdays, respectively.
Keywords: Cortical development; Intermediate progenitors; Laminar fate; MADM; Mosaic analysis with double markers; Tbr2.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Orderly specification and precise laminar deployment of mouse cortical projection neuron types through intermediate progenitors.Dev Cell. 2025 Jul 21;60(14):1947-1957.e3. doi: 10.1016/j.devcel.2025.02.009. Epub 2025 Mar 10. Dev Cell. 2025. PMID: 40068685
-
Intermediate progenitors and Tbr2 in cortical development.J Anat. 2019 Sep;235(3):616-625. doi: 10.1111/joa.12939. Epub 2019 Jan 24. J Anat. 2019. PMID: 30677129 Free PMC article. Review.
-
Neocortical Sox9+ radial glia generate glutamatergic neurons for all layers, but lack discernible evidence of early laminar fate restriction.Neural Dev. 2017 Aug 16;12(1):14. doi: 10.1186/s13064-017-0091-4. Neural Dev. 2017. PMID: 28814327 Free PMC article.
-
Intermediate Progenitor Cohorts Differentially Generate Cortical Layers and Require Tbr2 for Timely Acquisition of Neuronal Subtype Identity.Cell Rep. 2016 Jun 28;16(1):92-105. doi: 10.1016/j.celrep.2016.05.072. Epub 2016 Jun 16. Cell Rep. 2016. PMID: 27320921 Free PMC article.
-
From radial glia to pyramidal-projection neuron: transcription factor cascades in cerebral cortex development.Mol Neurobiol. 2006 Feb;33(1):33-50. doi: 10.1385/MN:33:1:033. Mol Neurobiol. 2006. PMID: 16388109 Review.
Cited by
-
Orderly specification and precise laminar deployment of mouse cortical projection neuron types through intermediate progenitors.Dev Cell. 2025 Jul 21;60(14):1947-1957.e3. doi: 10.1016/j.devcel.2025.02.009. Epub 2025 Mar 10. Dev Cell. 2025. PMID: 40068685
-
Transcriptional Regulators and Human-Specific/Primate-Specific Genes in Neocortical Neurogenesis.Int J Mol Sci. 2020 Jun 29;21(13):4614. doi: 10.3390/ijms21134614. Int J Mol Sci. 2020. PMID: 32610533 Free PMC article. Review.
-
Behavior and lineage progression of neural progenitors in the mammalian cortex.Curr Opin Neurobiol. 2021 Feb;66:144-157. doi: 10.1016/j.conb.2020.10.017. Epub 2020 Nov 20. Curr Opin Neurobiol. 2021. PMID: 33227588 Free PMC article. Review.
-
Molecular and cellular evolution of corticogenesis in amniotes.Cell Mol Life Sci. 2020 Apr;77(8):1435-1460. doi: 10.1007/s00018-019-03315-x. Epub 2019 Sep 28. Cell Mol Life Sci. 2020. PMID: 31563997 Free PMC article. Review.
-
The microcephaly-associated transcriptional regulator AUTS2 cooperates with Polycomb complex PRC2 to produce upper-layer neurons in mice.EMBO J. 2025 Mar;44(5):1354-1378. doi: 10.1038/s44318-024-00343-7. Epub 2025 Jan 15. EMBO J. 2025. PMID: 39815005 Free PMC article.
References
-
- Blaschke A. J., Staley K. and Chun J. (1996). Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 122, 1165-1174. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

