Human T-cell leukemia virus type 1 Gag domains have distinct RNA-binding specificities with implications for RNA packaging and dimerization
- PMID: 30217825
- PMCID: PMC6200928
- DOI: 10.1074/jbc.RA118.005531
Human T-cell leukemia virus type 1 Gag domains have distinct RNA-binding specificities with implications for RNA packaging and dimerization
Abstract
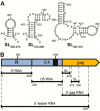
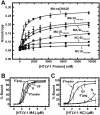
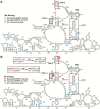
Human T-cell leukemia virus type 1 (HTLV-1) is the first retrovirus that has conclusively been shown to cause human diseases. In HIV-1, specific interactions between the nucleocapsid (NC) domain of the Gag protein and genomic RNA (gRNA) mediate gRNA dimerization and selective packaging; however, the mechanism for gRNA packaging in HTLV-1, a deltaretrovirus, is unclear. In other deltaretroviruses, the matrix (MA) and NC domains of Gag are both involved in gRNA packaging, but MA binds nucleic acids with higher affinity and has more robust chaperone activity, suggesting that this domain may play a primary role. Here, we show that the MA domain of HTLV-1, but not the NC domain, binds short hairpin RNAs derived from the putative gRNA packaging signal. RNA probing of the HTLV-1 5' leader and cross-linking studies revealed that the primer-binding site and a region within the putative packaging signal form stable hairpins that interact with MA. In addition to a previously identified palindromic dimerization initiation site (DIS), we identified a new DIS in HTLV-1 gRNA and found that both palindromic sequences bind specifically the NC domain. Surprisingly, a mutant partially defective in dimer formation in vitro exhibited a significant increase in RNA packaging into HTLV-1-like particles, suggesting that efficient RNA dimerization may not be strictly required for RNA packaging in HTLV-1. Moreover, the lifecycle of HTLV-1 and other deltaretroviruses may be characterized by NC and MA functions that are distinct from those of the corresponding HIV-1 proteins, but together provide the functions required for viral replication.
Keywords: Gag; HTLV-1; RNA binding protein; RNA dimerization; RNA folding; RNA structure; matrix domain; nucleocapsid; retrovirus; viral replication.
© 2018 Wu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Retrovirus-specific differences in matrix and nucleocapsid protein-nucleic acid interactions: implications for genomic RNA packaging.J Virol. 2014 Jan;88(2):1271-80. doi: 10.1128/JVI.02151-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227839 Free PMC article.
-
Distinct binding interactions of HIV-1 Gag to Psi and non-Psi RNAs: implications for viral genomic RNA packaging.RNA. 2013 Aug;19(8):1078-88. doi: 10.1261/rna.038869.113. Epub 2013 Jun 24. RNA. 2013. PMID: 23798665 Free PMC article.
-
The matrix domain contributes to the nucleic acid chaperone activity of HIV-2 Gag.Retrovirology. 2016 Mar 17;13:18. doi: 10.1186/s12977-016-0245-1. Retrovirology. 2016. PMID: 26987314 Free PMC article.
-
Retroviral Gag protein-RNA interactions: Implications for specific genomic RNA packaging and virion assembly.Semin Cell Dev Biol. 2019 Feb;86:129-139. doi: 10.1016/j.semcdb.2018.03.015. Epub 2018 Apr 1. Semin Cell Dev Biol. 2019. PMID: 29580971 Free PMC article. Review.
-
On the Selective Packaging of Genomic RNA by HIV-1.Viruses. 2016 Sep 12;8(9):246. doi: 10.3390/v8090246. Viruses. 2016. PMID: 27626441 Free PMC article. Review.
Cited by
-
Molecular Biology and Diversification of Human Retroviruses.Front Virol. 2022;2:872599. doi: 10.3389/fviro.2022.872599. Epub 2022 Jun 2. Front Virol. 2022. PMID: 35783361 Free PMC article.
-
Elements in the 5' Untranslated Region of Viral RNA Important for HIV Gag Recognition and Cross-Packaging.Viruses. 2025 Apr 10;17(4):551. doi: 10.3390/v17040551. Viruses. 2025. PMID: 40284994 Free PMC article.
-
Portrait of the Intrinsically Disordered Side of the HTLV-1 Proteome.ACS Omega. 2019 Jun 7;4(6):10003-10018. doi: 10.1021/acsomega.9b01017. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460093 Free PMC article.
-
Human T-cell leukemia virus type 1 uses a specific tRNAPro isodecoder to prime reverse transcription.RNA. 2024 Jul 16;30(8):967-976. doi: 10.1261/rna.080006.124. RNA. 2024. PMID: 38684316 Free PMC article.
-
Human RPL7 and DDX21 interact with HTLV-1 Gag and enhance tRNAPro primer annealing to genomic RNA.bioRxiv [Preprint]. 2025 Jul 15:2025.07.15.664966. doi: 10.1101/2025.07.15.664966. bioRxiv. 2025. PMID: 40791341 Free PMC article. Preprint.
References
-
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., and Gallo R. C. (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U.S.A. 77, 7415–7419 10.1073/pnas.77.12.7415 - DOI - PMC - PubMed
-
- Tsukasaki K., Hermine O., Bazarbachi A., Ratner L., Ramos J. C., Harrington W. Jr., O'Mahony D., Janik J. E., Bittencourt A. L., Taylor G. P., Yamaguchi K., Utsunomiya A., Tobinai K., and Watanabe T. (2009) Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J. Clin. Oncol. 27, 453–459 10.1200/JCO.2008.18.2428 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

