Phage-Antibiotic Synergy via Delayed Lysis
- PMID: 30217844
- PMCID: PMC6210123
- DOI: 10.1128/AEM.02085-18
Phage-Antibiotic Synergy via Delayed Lysis
Abstract
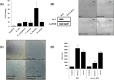
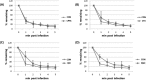
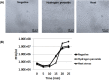
When phages infect bacteria cultured in the presence of sublethal doses of antibiotics, the sizes of the phage plaques are significantly increased. This phenomenon is known as phage-antibiotic synergy (PAS). In this study, the observation of PAS was extended to a wide variety of bacterium-phage pairs using different classes of antibiotics. PAS was shown in both Gram-positive and Gram-negative bacteria. Cells stressed with β-lactam antibiotics filamented or swelled extensively, resulting in an increase in phage production. PAS was also sometimes observed in the presence of other classes of antibiotics with or without bacterial filamentation. The addition of antibiotics induced recA expression in various bacteria, but a recA deletion mutant strain of Escherichia coli also showed filamentation and PAS in the presence of quinolone antibiotics. The phage adsorption efficiency did not change in the presence of the antibiotics when the cell surfaces were enlarged as they filamented. Increases in the production of phage DNA and mRNAs encoding phage proteins were observed in these cells, with only a limited increase in protein production. The data suggest that PAS is the product of a prolonged period of particle assembly due to delayed lysis. The increase in the cell surface area far exceeded the increase in phage holin production in the filamented host cells, leading to a relatively limited availability of intracellular holins for aggregating and forming holes in the host membrane. Reactive oxygen species (ROS) stress also led to an increased production of phages, while heat stress resulted in only a limited increase in phage production.IMPORTANCE Phage-antibiotic synergy (PAS) has been reported for a decade, but the underlying mechanism has never been vigorously investigated. This study shows the presence of PAS from a variety of phage-bacterium-antibiotic pairings. We show that increased phage production resulted directly from a lysis delay caused by the relative shortage of holin in filamented bacterial hosts in the presence of sublethal concentrations of stress-inducing substances, such as antibiotics and reactive oxygen species (ROS).
Keywords: filamentation; holin; lysis delay; phage-antibiotic synergy; recA; stress.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Islam MR, Ogura Y, Asadulghani M, Ooka T, Murase K, Gotoh Y, Hayashi T. 2012. A sensitive and simple plaque formation method for the Stx2 phage of Escherichia coli O157:H7, which does not form plaques in the standard plating procedure. Plasmid 67:227–235. doi: 10.1016/j.plasmid.2011.12.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

