Catalase Expression in Azospirillum brasilense Sp7 Is Regulated by a Network Consisting of OxyR and Two RpoH Paralogs and Including an RpoE1→RpoH5 Regulatory Cascade
- PMID: 30217849
- PMCID: PMC6238060
- DOI: 10.1128/AEM.01787-18
Catalase Expression in Azospirillum brasilense Sp7 Is Regulated by a Network Consisting of OxyR and Two RpoH Paralogs and Including an RpoE1→RpoH5 Regulatory Cascade
Abstract
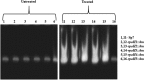
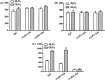
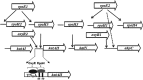
The genome of Azospirillum brasilense encodes five RpoH sigma factors: two OxyR transcription regulators and three catalases. The aim of this study was to understand the role they play during oxidative stress and their regulatory interconnection. Out of the 5 paralogs of RpoH present in A. brasilense, inactivation of only rpoH1 renders A. brasilense heat sensitive. While transcript levels of rpoH1 were elevated by heat stress, those of rpoH3 and rpoH5 were upregulated by H2O2 Catalase activity was upregulated in A. brasilense and its rpoH::km mutants in response to H2O2 except in the case of the rpoH5::km mutant, suggesting a role for RpoH5 in regulating inducible catalase. Transcriptional analysis of the katN, katAI, and katAII genes revealed that the expression of katN and katAII was severely compromised in the rpoH3::km and rpoH5::km mutants, respectively. Regulation of katN and katAII by RpoH3 and RpoH5, respectively, was further confirmed in an Escherichia coli two-plasmid system. Regulation of katAII by OxyR2 was evident by a drastic reduction in growth, KatAII activity, and katAII::lacZ expression in an oxyR2::km mutant. This study reports the involvement of RpoH3 and RpoH5 sigma factors in regulating oxidative stress response in alphaproteobacteria. We also report the regulation of an inducible catalase by a cascade of alternative sigma factors and an OxyR. Out of the three catalases in A. brasilense, those corresponding to katN and katAII are regulated by RpoH3 and RpoH5, respectively. The expression of katAII is regulated by a cascade of RpoE1→RpoH5 and OxyR2.IMPORTANCEIn silico analysis of the A. brasilense genome showed the presence of multiple paralogs of genes involved in oxidative stress response, which included 2 OxyR transcription regulators and 3 catalases. So far, Deinococcus radiodurans and Vibrio cholerae are known to harbor two paralogs of OxyR, and Sinorhizobium meliloti harbors three catalases. We do not yet know how the expression of multiple catalases is regulated in any bacterium. Here we show the role of multiple RpoH sigma factors and OxyR in regulating the expression of multiple catalases in A. brasilense Sp7. Our work gives a glimpse of systems biology of A. brasilense used for responding to oxidative stress.
Keywords: cascade; catalase; paralogs; sigma factor RpoH; transcriptional regulator OxyR.
Copyright © 2018 American Society for Microbiology.
Figures


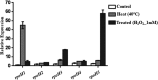





Similar articles
-
Carotenoid Biosynthetic Pathways Are Regulated by a Network of Multiple Cascades of Alternative Sigma Factors in Azospirillum brasilense Sp7.J Bacteriol. 2016 Oct 7;198(21):2955-2964. doi: 10.1128/JB.00460-16. Print 2016 Nov 1. J Bacteriol. 2016. PMID: 27551017 Free PMC article.
-
OxyR-Dependent Transcription Response of Sinorhizobium meliloti to Oxidative Stress.J Bacteriol. 2018 Mar 12;200(7):e00622-17. doi: 10.1128/JB.00622-17. Print 2018 Apr 1. J Bacteriol. 2018. PMID: 29358497 Free PMC article.
-
Expression of alkyl hydroperoxide reductase is regulated negatively by OxyR1 and positively by RpoE2 sigma factor in Azospirillum brasilense Sp7.Microbiology (Reading). 2016 Oct;162(10):1870-1883. doi: 10.1099/mic.0.000363. Epub 2016 Aug 23. Microbiology (Reading). 2016. PMID: 27557935
-
Transcriptional regulation of stress-inducible genes in procaryotes.EXS. 1996;77:165-81. doi: 10.1007/978-3-0348-9088-5_11. EXS. 1996. PMID: 8856974 Review.
-
Regulation of hydroperoxidase (catalase) expression in Escherichia coli.FEMS Microbiol Lett. 1995 Sep 1;131(2):113-9. doi: 10.1111/j.1574-6968.1995.tb07764.x. FEMS Microbiol Lett. 1995. PMID: 7557318 Review.
Cited by
-
Cometabolism of Ethanol in Azospirillum brasilense Sp7 Is Mediated by Fructose and Glycerol and Regulated Negatively by an Alternative Sigma Factor RpoH2.J Bacteriol. 2021 Nov 19;203(24):e0026921. doi: 10.1128/JB.00269-21. Epub 2021 Sep 27. J Bacteriol. 2021. PMID: 34570625 Free PMC article.
-
β-Lactam Resistance in Azospirillum baldaniorum Sp245 Is Mediated by Lytic Transglycosylase and β-Lactamase and Regulated by a Cascade of RpoE7→RpoH3 Sigma Factors.J Bacteriol. 2022 Apr 19;204(4):e0001022. doi: 10.1128/jb.00010-22. Epub 2022 Mar 30. J Bacteriol. 2022. PMID: 35352964 Free PMC article.
-
Editorial: Role of transcription factors and sigma factors in bacterial stress physiology.Front Microbiol. 2023 Oct 6;14:1291172. doi: 10.3389/fmicb.2023.1291172. eCollection 2023. Front Microbiol. 2023. PMID: 37869661 Free PMC article. No abstract available.
-
Characterization of Hsp17, a Novel Small Heat Shock Protein, in Sphingomonas melonis TY under Heat Stress.Microbiol Spectr. 2023 Aug 17;11(4):e0136023. doi: 10.1128/spectrum.01360-23. Epub 2023 Jul 12. Microbiol Spectr. 2023. PMID: 37436164 Free PMC article.
-
Redox Regulation in Diazotrophic Bacteria in Interaction with Plants.Antioxidants (Basel). 2021 May 30;10(6):880. doi: 10.3390/antiox10060880. Antioxidants (Basel). 2021. PMID: 34070926 Free PMC article. Review.
References
-
- de Bruijn FJ, Biran D, Ron EZ, Van Nostrand JD, Zhou A, Zhou J, Ishihama A, Sharma UK, Jin DJ, Cagliero C, Izard J. 2016. Stress and environmental regulation of gene expression and adaptation in bacteria. Wiley-Blackwell, Hoboken, NJ.
-
- Gross CA, Lonetto M, Losick R. 1992. Bacterial sigma factors, p 129–176. In McKnight SL, Yamamoto KR (ed), Transcriptional regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

