Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier
- PMID: 30217895
- PMCID: PMC6176561
- DOI: 10.1073/pnas.1802155115
Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier
Abstract
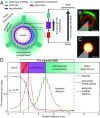
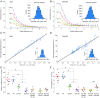
The endothelial cells that form the blood-brain barrier (BBB) are coated with glycocalyx, on the luminal side, and with the basement membrane and astrocyte endfeet, on the abluminal side. However, it is unclear how exactly the glycocalyx and extravascular structures contribute to BBB properties. We used two-photon microscopy in anesthetized mice to record passive transport of four different-sized molecules-sodium fluorescein (376 Da), Alexa Fluor (643 Da), 40-kDa dextran, and 150-kDa dextran-from blood to brain, at the level of single cortical capillaries. Both fluorescein and Alexa penetrated nearly the entire glycocalyx volume, but the dextrans penetrated less than 60% of the volume. This suggested that the glycocalyx was a barrier for large but not small molecules. The estimated permeability of the endothelium was the same for fluorescein and Alexa but several-fold lower for the larger dextrans. In the extravascular compartment, co-localized with astrocyte endfeet, diffusion coefficients of the dyes were an order of magnitude lower than in the brain parenchyma. This suggested that the astrocyte endfeet and basement membrane also contributed to BBB properties. In conclusion, the passive transport of small and large hydrophilic molecules through the BBB was determined by three separate barriers: the glycocalyx, the endothelium, and the extravascular compartment. All three barriers must be taken into account in drug delivery studies and when considering BBB dysfunction in disease states.
Keywords: astrocytes; blood–brain barrier; diffusion; glycocalyx; permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fenstermacher JD, Blasberg RG, Patlak CS. Methods for quantifying the transport of drugs across brain barrier systems. Pharmacol Ther. 1981;14:217–248. - PubMed
-
- Gjedde A. High- and low-affinity transport of D-glucose from blood to brain. J Neurochem. 1981;36:1463–1471. - PubMed
-
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. - PubMed
-
- Blasberg RG, Patlak CS, Fenstermacher JD. Selection of experimental conditions for the accurate determination of blood—Brain transfer constants from single-time experiments: A theoretical analysis. J Cereb Blood Flow Metab. 1983;3:215–225. - PubMed
-
- Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: Alpha-lactalbumin transport. Am J Physiol. 1987;252:H188–H197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

