Interleukin-22 promotes phagolysosomal fusion to induce protection against Salmonella enterica Typhimurium in human epithelial cells
- PMID: 30217896
- PMCID: PMC6176607
- DOI: 10.1073/pnas.1811866115
Interleukin-22 promotes phagolysosomal fusion to induce protection against Salmonella enterica Typhimurium in human epithelial cells
Abstract
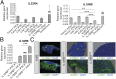
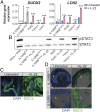
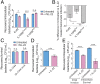
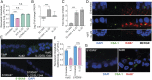
Intestinal epithelial cells (IECs) play a key role in regulating immune responses and controlling infection. However, the direct role of IECs in restricting pathogens remains incompletely understood. Here, we provide evidence that IL-22 primed intestinal organoids derived from healthy human induced pluripotent stem cells (hIPSCs) to restrict Salmonella enterica serovar Typhimurium SL1344 infection. A combination of transcriptomics, bacterial invasion assays, and imaging suggests that IL-22-induced antimicrobial activity is driven by increased phagolysosomal fusion in IL-22-pretreated cells. The antimicrobial phenotype was absent in hIPSCs derived from a patient harboring a homozygous mutation in the IL10RB gene that inactivates the IL-22 receptor but was restored by genetically complementing the IL10RB deficiency. This study highlights a mechanism through which the IL-22 pathway facilitates the human intestinal epithelium to control microbial infection.
Keywords: Salmonella; interleukin-22; intestinal organoids.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

