Role of phosphate sensing in bone and mineral metabolism
- PMID: 30218014
- PMCID: PMC8607960
- DOI: 10.1038/s41574-018-0076-3
Role of phosphate sensing in bone and mineral metabolism
Abstract
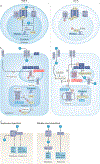
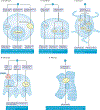
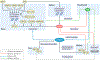
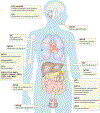
Inorganic phosphate (Pi) is essential for signal transduction and cell metabolism, and is also an essential structural component of the extracellular matrix of the skeleton. Pi is sensed in bacteria and yeast at the plasma membrane, which activates intracellular signal transduction to control the expression of Pi transporters and other genes that control intracellular Pi levels. In multicellular organisms, Pi homeostasis must be maintained in the organism and at the cellular level, requiring an endocrine and metabolic Pi-sensing mechanism, about which little is currently known. This Review will discuss the metabolic effects of Pi, which are mediated by Pi transporters, inositol pyrophosphates and SYG1-Pho81-XPR1 (SPX)-domain proteins to maintain cellular phosphate homeostasis in the musculoskeletal system. In addition, we will discuss how Pi is sensed by the human body to regulate the production of fibroblast growth factor 23 (FGF23), parathyroid hormone and calcitriol to maintain serum levels of Pi in a narrow range. New findings on the crosstalk between iron and Pi homeostasis in the regulation of FGF23 expression will also be outlined. Mutations in components of these metabolic and endocrine phosphate sensors result in genetic disorders of phosphate homeostasis, cardiomyopathy and familial basal ganglial calcifications, highlighting the importance of this newly emerging area of research.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
References
-
- Bevington A., Kemp GJ., Graham R. & Russell G. Phosphate-sensitive enzymes: possible molecular basis for cellular disorders of phosphate metabolism. Clin. Chem. Enzym. Comms. 4, 235–257 (1992).
-
- Angelova PR, Baev AY, Berezhnov AV & Abramov AY Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 44, 40–45 (2016). - PubMed
-
- Herman H. & Dallemagne MJ The main mineral constituent of bone and teeth. Arch. Oral Biol. 5, 137–144 (1961). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

