N-Methyl Pyrrolidone (NMP) Alleviates Lipopolysaccharide (LPS)-Induced Inflammatory Injury in Articular Chondrocytes
- PMID: 30218608
- PMCID: PMC6151968
- DOI: 10.12659/MSM.910050
N-Methyl Pyrrolidone (NMP) Alleviates Lipopolysaccharide (LPS)-Induced Inflammatory Injury in Articular Chondrocytes
Abstract
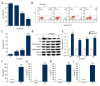
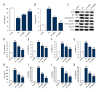
BACKGROUND Studies on the chondrocyte inflammatory injury are very important for understanding the pathogenesis and clinical treatment of osteoarthritis (OA). Evidence suggests that N-methyl pyrrolidone (NMP) may be used as an adjuvant therapy alongside established methods of OA treatment. This study investigated the effect of NMP on chondrocyte inflammatory injury and explored the underlying molecular mechanism. MATERIAL AND METHODS To mimic the inflammatory injury in vitro, the articular chondrocyte line ATDC5 was simulated with lipopolysaccharide (LPS). ATDC5 cells were treated with various concentrations of NMP (0, 5, and 10 nM). Cell viability was measured using CCK-8 assay; cell apoptosis was detected using FCM; related protein and mRNA expressions were determined using Western blot assay and qRT-PCR assay; and inflammatory factors (tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8) productions were measured by performing ELISA assay. RESULTS The results showed that LPS simulation repressed ATDC5 cell viability, prompted cell apoptosis, and enhanced the secretion of inflammatory factors. NMP treatment reduced inflammatory injury induced by LPS in a dose-dependent manner. Furthermore, NMP inhibited the activation of JNK and p38 pathways. In addition, inhibition of NF-κB activation was observed following NMP treatment. CONCLUSIONS NMP prevents inflammatory reaction of articular chondrocytes via repressing the MAPK/NF-kB pathway. Our findings provide a promising therapeutic agent for OA treatment.
Conflict of interest statement
None.
Figures
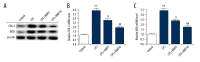


References
-
- Pelletier JP, Mantel-Pelletier JF, Malemud CJ. Immunological analysis of proteoglycan changes in the early stage of osteoarthritic canine cartilage lesions. J Orthop Res. 1992;10:511–23. - PubMed
-
- Colen S, Bekerom MPJVD, Mulier M. Intra-articular viscosupplementation in patients with hip osteoarthritis. Eur Musculoskelet Rev. 2011;6:79–82.
-
- Arden N, Blanco F, Cooper C, et al. Atlas of osteosteoarthritis. Springer Healthcare Ltd; 2014. pp. 69–82.
-
- Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34. - PubMed
-
- Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: Its prevalence and relationship to physical function. Arthritis Rheum. 2004;51(6):941–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

