Organoid technology and applications in cancer research
- PMID: 30219074
- PMCID: PMC6139148
- DOI: 10.1186/s13045-018-0662-9
Organoid technology and applications in cancer research
Abstract
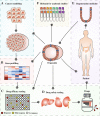
During the past decade, the three-dimensional organoid technology has sprung up and become more and more popular among researchers. Organoids are the miniatures of in vivo tissues and organs, and faithfully recapitulate the architectures and distinctive functions of a specific organ.These amazing three-dimensional constructs represent a promising, near-physiological model for human cancers, and tremendously support diverse potential applications in cancer research. Up to now, highly efficient establishment of organoids can be achieved from both normal and malignant tissues of patients. Using this bioengineered platform, the links of infection-cancer progression and mutation-carcinogenesis are feasible to be modeled. Another potential application is that organoid technology facilitates drug testing and guides personalized therapy. Although organoids still fail to model immune system accurately, co-cultures of organoids and lymphocytes have been reported in several studies, bringing hope for further application of this technology in immunotherapy. In addition, the potential value in regeneration medicine might be another paramount branch of organoid technology, which might refine current transplantation therapy through the replacement of irreversibly progressively diseased organs with isogenic healthy organoids.In conclusion, organoids represent an excellent preclinical model for human tumors, promoting the translation from basic cancer research to clinical practice. In this review, we outline organoid technology and summarize its applications in cancer research.
Keywords: Cancer; Drug development; Drug efficacy; Drug toxicity; Immunotherapy; Organoid; Personalized medicine; Regeneration medicine.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

