The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis
- PMID: 30219899
- PMCID: PMC6383060
- DOI: 10.1093/cvr/cvy215
The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis
Abstract
Aims: Single nucleotide polymorphisms on chromosome 4q25 have been associated with risk of atrial fibrillation (AF) but the exiguous knowledge of the mechanistic links between these risk variants and underlying electrophysiological alterations hampers their clinical utility. Here, we tested the hypothesis that 4q25 risk variants cause alterations in the intracellular calcium homoeostasis that predispose to spontaneous electrical activity.
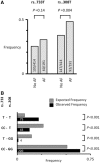
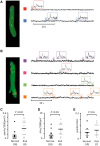
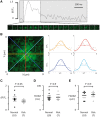
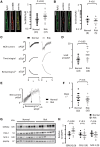
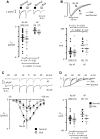
Methods and results: Western blotting, confocal calcium imaging, and patch-clamp techniques were used to identify mechanisms linking the 4q25 risk variants rs2200733T and rs13143308T to defects in the calcium homoeostasis in human atrial myocytes. Our findings revealed that the rs13143308T variant was more frequent in patients with AF and that myocytes from carriers of this variant had a significantly higher density of calcium sparks (14.1 ± 4.5 vs. 3.1 ± 1.3 events/min, P = 0.02), frequency of transient inward currents (ITI) (1.33 ± 0.24 vs. 0.26 ± 0.09 events/min, P < 0.001) and incidence of spontaneous membrane depolarizations (1.22 ± 0.26 vs. 0.56 ± 0.17 events/min, P = 0.001) than myocytes from patients with the normal rs13143308G variant. These alterations were linked to higher sarcoplasmic reticulum calcium loading (10.2 ± 1.4 vs. 7.3 ± 0.5 amol/pF, P = 0.01), SERCA2 expression (1.37 ± 0.13 fold, P = 0.03), and RyR2 phosphorylation at ser2808 (0.67 ± 0.08 vs. 0.47 ± 0.03, P = 0.01) but not at ser2814 (0.28 ± 0.14 vs. 0.31 ± 0.14, P = 0.61) in patients carrying the rs13143308T risk variant. Furthermore, the presence of a risk variant or AF independently increased the ITI frequency and the increase in the ITI frequency observed in carriers of the risk variants was exacerbated in those with AF. By contrast, the presence of a risk variant did not affect the amplitude or properties of the L-type calcium current in patients with or without AF.
Conclusions: Here, we identify the 4q25 variant rs13143308T as a genetic risk marker for AF, specifically associated with excessive calcium release and spontaneous electrical activity linked to increased SERCA2 expression and RyR2 phosphorylation.
Keywords: Human atrial myocytes; Ryanodine receptor; Sarcoplasmic reticulum calcium release; Single nucleotide polymorphisms; Spontaneous electrical activity.
© The Author(s) 2018. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Ménage à trois: single-nucleotide polymorphisms, calcium and atrial fibrillation.Cardiovasc Res. 2019 Mar 1;115(3):479-481. doi: 10.1093/cvr/cvy283. Cardiovasc Res. 2019. PMID: 30428015 No abstract available.
References
-
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P.. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. - PubMed
-
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA.. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. - PubMed
-
- Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J.. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 2004;110:1358–1363. - PubMed
-
- Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casado V, Ciruela F, Lluis C, Franco R, Cinca J, Hove-Madsen L.. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J 2011;32:721–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

