Multicenter, Open-Label Study of Long-Term Topiroxostat (FYX-051) Administration in Japanese Hyperuricemic Patients with or Without Gout
- PMID: 30219951
- PMCID: PMC6267543
- DOI: 10.1007/s40261-018-0699-0
Multicenter, Open-Label Study of Long-Term Topiroxostat (FYX-051) Administration in Japanese Hyperuricemic Patients with or Without Gout
Abstract
Background and objectives: Topiroxostat-a novel selective xanthine oxidoreductase inhibitor-has been reported to reduce serum urate levels. The purpose of this study was to assess the efficacy and safety of long-term topiroxostat administration in Japanese hyperuricemic patients with or without gout.
Methods: This multicenter, open-label study evaluated the efficacy and safety of long-term twice-daily oral topiroxostat administration in patients with or without gout. The initial topiroxostat dosage was 40-80 mg/day, and the maintenance dosage was 120 mg/day, which was increased to 240 mg/day at 40 mg increments if the serum urate level exceeded 6.0 mg/dL.
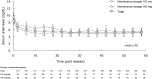
Results: Serum urate level, which was the primary endpoint, decreased stably over time and showed significant reduction on the final visit (38.44% ± 13.34%) compared with that at the baseline. Both urinary albumin/creatinine ratio and mean blood pressure significantly improved. The overall incidence rate of adverse drug reactions to topiroxostat was 67.8%; on the final visit, the rate of adverse drug reactions was 66.7% with 120 mg/day, 72.2% with 160 mg/day, 53.8% with ≥ 200 mg/day, and 100% with the other dosages. On the final visit, the incidence of gouty arthritis, for which a causal relationship with topiroxostat could not be ruled out, was 4.1% overall, 4.8% with 120 mg/day, 0% with 160 mg/day, and 7.7% with ≥ 200 mg/day.
Conclusions: We verified the efficacy and safety of 58-week oral topiroxostat administration at stepwise increments to up to 240 mg/day.
Study registration: JAPIC CTI-101068.
Conflict of interest statement
Conflict of interest
TH has received consultant fees and/or speakers’ honoraria from Fuji Yakuhin Co., Ltd., the manufacturer of topiroxostat, and/or Sanwa Kagaku Kenkyusho Co., Ltd. TI and TO are employees of Fuji Yakuhin Co., Ltd. RS and YO are employees of Sanwa Kagaku Kenkyusho Co., Ltd.
Ethics Approval
This study and its protocol were approved by the following local IRBs of participating sites: Chubu Rosai Hospital IRB (02/22/2010), IRB Of Kouseikai Sone Clinic (02/24/2010), Abe Clinic IRB (02/24/2010), Gifu Prefectural General Medical Center IRB (03/17/2010), Social Medical Corporation the Chiyukai foundation Fukuoka Wajiro Hospital IRB (03/18/2010). This study was conducted in compliance with the Helsinki Declaration, Good Clinical Practice guidelines, and other relevant laws and regulations.
Informed Consent
All patients provided written informed consent before initiation of the study.
Figures

References
-
- Khanna D, Fitzgerald J, Khanna P, Bae S, Singh M, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–1446. doi: 10.1002/acr.21772. - DOI - PMC - PubMed
-
- Sivera F, Andrés M, Carmona L, Kydd A, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73:328–335. doi: 10.1136/annrheumdis-2013-203325. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

