Afamin: an early predictor of preeclampsia
- PMID: 30220025
- PMCID: PMC6182689
- DOI: 10.1007/s00404-018-4897-z
Afamin: an early predictor of preeclampsia
Abstract
Purpose: Oxidative stress is involved in the pathogenesis of hypertensive disorders such as preeclampsia (PE) and associated with the human vitamin E-binding protein afamin. The aim of this study was, therefore, to analyse afamin in the first trimester of patients developing PE later in pregnancy and in control subjects without pregnancy complications.
Methods: In this retrospective study, 137 serum samples from the first trimester of pregnancy were analysed in a case-control study design. 39 patients developed PE (10 patients with early-onset and 29 patients with late onset disease) and 98 women had an uncomplicated pregnancy. Mann-Whitney U test, t test, logistic regression and ROC analyses were performed for statistical evaluation.
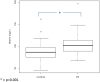
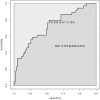
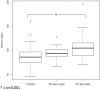
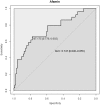
Results: Pregnant women developing PE presented with higher afamin concentrations in the first trimester [median 101.81 mg/L; interquartile range (IQR) 88.94-113.26] compared to subjects with uncomplicated pregnancy (median 86.40; IQR 75.26-96.92; p < 0.001). After adjusting for confounders, the odds ratio per afamin standard deviation was 1.60 (95% CI: 1.04-2.58; p = 0.04). An afamin threshold concentration of 87.8 mg/L exhibited the best sensitivity (79.5%) and specificity (57.1%) in predicting PE. Subgroup analysis of early- and late-onset disease resulted in substantially higher afamin concentrations in women with developing late-onset PE compared to controls (p < 0.001) with an odds ratio per afamin standard deviation of 1.62 (95% CI: 0.98-2.70; p = 0.06).
Conclusions: Serum afamin concentrations are elevated in the first trimester among patients developing PE compared to controls. Substantial differences were observed mainly among patients with late-onset PE.
Keywords: Afamin; Early and late onset; Prediction; Preeclampsia.
Conflict of interest statement
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
This study was approved by the research ethics committee of the University of Duisburg-Essen (number 125212-BO) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All women provided written informed consent.
Figures
References
-
- Purde MT, Baumann M, Wiedemann U, Nydegger UE, Risch L, Surbek D, Risch M. Incidence of preeclampsia in pregnant Swiss women. Swiss Med Wkly. 2015;145:w14175. - PubMed
-
- Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, Leipold G, Akolekar R, Shearing S, De Stefani L, Jani JC, Plasencia W, Evangelinakis N, Gonzalez-Vanegas O, Persico N, Nicolaides KH. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217:585. doi: 10.1016/j.ajog.2017.07.038. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

