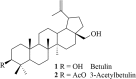
Novel triazoles of 3-acetylbetulin and betulone as anticancer agents
- PMID: 30220830
- PMCID: PMC6133159
- DOI: 10.1007/s00044-018-2213-x
Novel triazoles of 3-acetylbetulin and betulone as anticancer agents
Abstract
The CuAAC reaction of azides and acetylenic triterpenes was used for synthesis of new triazoles of 3-acetylbetulin and betulone. The triazole derivatives were evaluated for their anticancer activity in vitro against amelanotic melanoma C-32, ductal carcinoma T47D and glioblastoma SNB-19 cell lines. 28-[1-(3'-Deoxythymidine-5'-yl)-1H-1,2,3-triazol-4-yl]carbonylbetulone 6e exhibited a significant IC50 value (0.17 µM) against the human glioblastoma SNB-19 cell line, an almost 5-fold higher potency while compared with reference cisplatin.
Keywords: 1,2,3-Triazole; Anticancer activity; Betulin; CuAAC; Lipophilicity.
Conflict of interest statement
Compliance with ethical standardsThe authors declare that they have no conflict of interest.
Figures
Similar articles
-
Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile.Molecules. 2017 Nov 1;22(11):1876. doi: 10.3390/molecules22111876. Molecules. 2017. PMID: 29104263 Free PMC article.
-
New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity.Med Chem Res. 2017;26(1):1-8. doi: 10.1007/s00044-016-1713-9. Epub 2016 Sep 1. Med Chem Res. 2017. PMID: 28111514 Free PMC article. Review.
-
Novel 3'-[4-fluoroaryl-(1,2,3-triazol-1-yl)]-3'-deoxythymidine analogues: Design, synthesis, characterization and their potential as anticancer agents.Nucleosides Nucleotides Nucleic Acids. 2022;41(4):343-360. doi: 10.1080/15257770.2022.2029883. Epub 2022 Jan 30. Nucleosides Nucleotides Nucleic Acids. 2022. PMID: 35094645
-
Molecular Structure, In Vitro Anticancer Study and Molecular Docking of New Phosphate Derivatives of Betulin.Molecules. 2021 Jan 31;26(3):737. doi: 10.3390/molecules26030737. Molecules. 2021. PMID: 33572631 Free PMC article.
-
Cu (I) catalyzed alkyne-azide 1,3-dipolar cycloaddition (CuAAC): Synthesis of 17α-[1-(substituted phenyl)-1,2,3-triazol-4-yl]-19-nor-testosterone-17β-yl acetates targeting progestational and antipro-liferative activities.Eur J Med Chem. 2015 Jun 5;97:75-82. doi: 10.1016/j.ejmech.2015.04.045. Epub 2015 Apr 28. Eur J Med Chem. 2015. PMID: 25942354
Cited by
-
Supported Silver Nanoparticles as Catalysts for Liquid-Phase Betulin Oxidation.Nanomaterials (Basel). 2021 Feb 12;11(2):469. doi: 10.3390/nano11020469. Nanomaterials (Basel). 2021. PMID: 33673079 Free PMC article.
-
Click Chemistry: an overview and recent updates in the medicinal attributes of click-derived heterocycles.Mol Divers. 2025 Feb 7. doi: 10.1007/s11030-025-11110-z. Online ahead of print. Mol Divers. 2025. PMID: 39918713 Review.
-
Bile-Acid-Appended Triazolyl Aryl Ketones: Design, Synthesis, In Vitro Anticancer Activity and Pharmacokinetics in Rats.Molecules. 2021 Sep 22;26(19):5741. doi: 10.3390/molecules26195741. Molecules. 2021. PMID: 34641285 Free PMC article.
-
1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview.Bioorg Med Chem. 2019 Aug 15;27(16):3511-3531. doi: 10.1016/j.bmc.2019.07.005. Epub 2019 Jul 4. Bioorg Med Chem. 2019. PMID: 31300317 Free PMC article. Review.
-
Synthesis and Anticancer Activity of Indole-Functionalized Derivatives of Betulin.Pharmaceutics. 2022 Nov 4;14(11):2372. doi: 10.3390/pharmaceutics14112372. Pharmaceutics. 2022. PMID: 36365190 Free PMC article.
References
-
- Bonacorso HG, Moraes MC, Wiethan CW, Luz FM, Meyer AR, Zanatta N, Martins MAP. Synthesis of 1H-1,2,3-triazoles-Rufinamide analogs by 1,3-dipolar cycloaddition and eletrocyclization reactions of trifluoroacetyl enolethers under thermal solventless conditions. J Flu Chem. 2013;156:112–119. doi: 10.1016/j.jfluchem.2013.09.005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources