Randomized study of doxorubicin-based chemotherapy regimens, with and without sildenafil, with analysis of intermediate cardiac markers
- PMID: 30221011
- PMCID: PMC6136838
- DOI: 10.1186/s40959-018-0033-2
Randomized study of doxorubicin-based chemotherapy regimens, with and without sildenafil, with analysis of intermediate cardiac markers
Abstract
Background: Doxorubicin chemotherapy is used across a range of adult and pediatric malignancies. Cardiac toxicity is common, and dysfunction develops over time in many patients. Biomarkers used for predicting late cardiac dysfunction following doxorubicin exposure have shown promise. Preclinical studies have demonstrated potential cardioprotective effects of sildenafil.
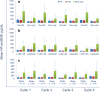
Methods: We sought to confirm the safety of adding sildenafil to doxorubicin-based chemotherapy and assess N-terminal Pro-Brain Natriuretic Peptide (NT-proBNP) and high sensitivity cardiac troponin I (hsTnI) as early markers of anthracycline-induced cardiotoxicity. We randomized 27 patients (ages 31-77, 92.3% female) receiving doxorubicin chemotherapy using a blocked randomization scheme with randomly permuted block sizes to receive standard chemotherapy alone or with the addition of sildenafil. The study was not blinded. Sildenafil was dosed at 100 mg by mouth daily during therapy; patients took sildenafil three times daily on the day of doxorubicin. Doxorubicin dosing and schedule were dependent on the treatment regimen. Echocardiography was obtained prior to initiation of treatment and routinely thereafter up to 4 years. NT-proBNP and hsTnI were obtained with each cycle before, 1-3 h after, and 24 h after doxorubicin.
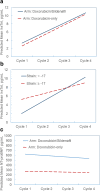
Results: Fourteen patients were randomized to receive standard doxorubicin chemotherapy alone (14 treated and analyzed), while 13 patients were randomized to the experimental doxorubicin and sildenafil arm (10 treated and analyzed). No toxicity signal was seen with the addition of sildenafil to doxorubicin-based regimens. There was no statistical difference between the treatment arms in relation to the change of mean left ventricular ejection fraction (LVEF) between the first and last evaluation. In both arms, hsTnI levels increased over time; however, elevated hsTnI was not associated with declines in LVEF.
Conclusion: Adding sildenafil was safe, but did not offer cardioprotection following doxorubicin treatment. Increases in hsTnI levels were observed over time, but elevations during therapy did not correlate with subsequent decreases in LVEF.
Trial registration: This clinical trial (NCT01375699) was registered at www.clinicaltrials.gov on June 17, 2011.
Keywords: Anthracycline; Biomarker; Cardioprotection; Chemotherapy; Clinical trial; Doxorubicin; Ejection fraction; Strain.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests.
Figures


Similar articles
-
Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction.Asia Pac J Clin Oncol. 2013 Jun;9(2):155-61. doi: 10.1111/j.1743-7563.2012.01588.x. Epub 2012 Aug 16. Asia Pac J Clin Oncol. 2013. PMID: 22897825
-
Use of N-terminal pro-brain natriuretic peptide to assess left ventricular function after adjuvant doxorubicin therapy in early breast cancer patients: a prospective series.Clin Drug Investig. 2009;29(2):131-7. doi: 10.2165/0044011-200929020-00007. Clin Drug Investig. 2009. PMID: 19133708
-
Combination of high-sensitivity troponin I and N-terminal pro-B-type natriuretic peptide predicts future hospital admission for heart failure in high-risk hypertensive patients with preserved left ventricular ejection fraction.Heart Vessels. 2017 Jul;32(7):880-892. doi: 10.1007/s00380-017-0948-9. Epub 2017 Feb 2. Heart Vessels. 2017. PMID: 28154958
-
Cardiotoxic Effect of Modern Anthracycline Dosing on Left Ventricular Ejection Fraction: A Systematic Review and Meta-Analysis of Placebo Arms From Randomized Controlled Trials.J Am Heart Assoc. 2021 Mar 16;10(6):e018802. doi: 10.1161/JAHA.120.018802. Epub 2021 Mar 4. J Am Heart Assoc. 2021. PMID: 33660514 Free PMC article.
-
Dexrazoxane. A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy.Drugs. 1998 Sep;56(3):385-403. doi: 10.2165/00003495-199856030-00009. Drugs. 1998. PMID: 9777314 Review.
Cited by
-
A pilot phase Ib study to evaluate tadalafil to overcome immunosuppression during chemoradiotherapy for IDH-wild-type glioblastoma.Neurooncol Adv. 2023 Jul 19;5(1):vdad088. doi: 10.1093/noajnl/vdad088. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37554225 Free PMC article.
-
Pros and Cons of Pharmacological Manipulation of cGMP-PDEs in the Prevention and Treatment of Breast Cancer.Int J Mol Sci. 2021 Dec 27;23(1):262. doi: 10.3390/ijms23010262. Int J Mol Sci. 2021. PMID: 35008687 Free PMC article. Review.
-
Rethinking of phosphodiesterase 5 inhibition: the old, the new and the perspective in human health.Front Endocrinol (Lausanne). 2024 Sep 17;15:1461642. doi: 10.3389/fendo.2024.1461642. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39355618 Free PMC article. Review.
-
Evidence-based prediction and prevention of cardiovascular morbidity in adults treated for cancer.Cardiooncology. 2021 May 28;7(1):20. doi: 10.1186/s40959-021-00105-y. Cardiooncology. 2021. PMID: 34049593 Free PMC article. Review.
-
The Potential Role of Sildenafil in Cancer Management through EPR Augmentation.J Pers Med. 2021 Jun 21;11(6):585. doi: 10.3390/jpm11060585. J Pers Med. 2021. PMID: 34205602 Free PMC article. Review.
References
-
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. - DOI - PubMed
-
- FDA Statement on Dexrazoxane. In.: U.S. Food and Drug Administration. Drug Saf and Availability; 2011.
-
- Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock G, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–497. doi: 10.1016/S0140-6736(16)30587-6. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
