γ-tubulin as a signal-transducing molecule and meshwork with therapeutic potential
- PMID: 30221013
- PMCID: PMC6137058
- DOI: 10.1038/s41392-018-0021-x
γ-tubulin as a signal-transducing molecule and meshwork with therapeutic potential
Abstract
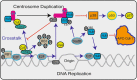
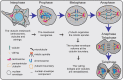
Knowledge of γ-tubulin is increasing with regard to the cellular functions of this protein beyond its participation in microtubule nucleation. γ-Tubulin expression is altered in various malignancies, and changes in the TUBG1 gene have been found in patients suffering from brain malformations. This review recapitulates the known functions of γ-tubulin in cellular homeostasis and discusses the possible influence of the protein on disease development and cancer.
Conflict of interest statement
The author declares no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

