1-Aminobenzotriazole: A Mechanism-Based Cytochrome P450 Inhibitor and Probe of Cytochrome P450 Biology
- PMID: 30221034
- PMCID: PMC6137267
- DOI: 10.4172/2161-0444.1000495
1-Aminobenzotriazole: A Mechanism-Based Cytochrome P450 Inhibitor and Probe of Cytochrome P450 Biology
Abstract
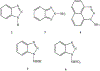
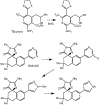
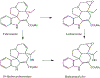
1-Aminobenzotriazole (1-ABT) is a pan-specific, mechanism-based inactivator of the xenobiotic metabolizing forms of cytochrome P450 in animals, plants, insects, and microorganisms. It has been widely used to investigate the biological roles of cytochrome P450 enzymes, their participation in the metabolism of both endobiotics and xenobiotics, and their contributions to the metabolism-dependent toxicity of drugs and chemicals. This review is a comprehensive evaluation of the chemistry, discovery, and use of 1-aminobenzotriazole in these contexts from its introduction in 1981 to the present.
Keywords: 1-Aminobenzotriazole; Arachidonic acid oxidation; Benzyne; Cytochrome P450; Drug metabolism; Estradiol; Heme adducts; Mechanism-based inhibition; Xenobiotics.
Conflict of interest statement
Disclosure Statement The author reports no conflicts of interest.
Figures
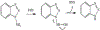
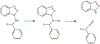
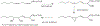
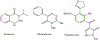
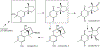
Similar articles
-
Inhibition of adrenal cytochromes P450 by 1-aminobenzotriazole in vitro. Selectivity for xenobiotic metabolism.Biochem Pharmacol. 1994 Oct 7;48(7):1421-6. doi: 10.1016/0006-2952(94)90566-5. Biochem Pharmacol. 1994. PMID: 7945442
-
Oxidation of endobiotics mediated by xenobiotic-metabolizing forms of human cytochrome.Curr Drug Metab. 2009 Sep;10(7):700-12. doi: 10.2174/138920009789895525. Curr Drug Metab. 2009. PMID: 19594405 Review.
-
Methoxsalen as an in vitro phenotyping tool in comparison with 1-aminobenzotriazole.Xenobiotica. 2019 Feb;49(2):169-176. doi: 10.1080/00498254.2018.1434913. Epub 2018 Feb 15. Xenobiotica. 2019. PMID: 29382249
-
Case Study 9: Probe-Dependent Binding Explains Lack of CYP2C9 Inactivation by 1-Aminobenzotriazole (ABT).Methods Mol Biol. 2021;2342:765-779. doi: 10.1007/978-1-0716-1554-6_28. Methods Mol Biol. 2021. PMID: 34272716
-
Emerging roles for brain drug-metabolizing cytochrome P450 enzymes in neuropsychiatric conditions and responses to drugs.Drug Metab Rev. 2016 Aug;48(3):379-404. doi: 10.1080/03602532.2016.1221960. Drug Metab Rev. 2016. PMID: 27498925 Review.
Cited by
-
Transcriptome analysis and cytochrome P450 monooxygenase reveal the molecular mechanism of Bisphenol A degradation by Pseudomonas putida strain YC-AE1.BMC Microbiol. 2022 Dec 9;22(1):294. doi: 10.1186/s12866-022-02689-6. BMC Microbiol. 2022. PMID: 36482332 Free PMC article.
-
The identification of dual protective agents against cisplatin-induced oto- and nephrotoxicity using the zebrafish model.Elife. 2020 Jul 28;9:e56235. doi: 10.7554/eLife.56235. Elife. 2020. PMID: 32720645 Free PMC article.
-
Resistance to dicyclanil and imidacloprid in the sheep blowfly, Lucilia cuprina, in Australia.Pest Manag Sci. 2022 Oct;78(10):4195-4206. doi: 10.1002/ps.7037. Epub 2022 Jul 2. Pest Manag Sci. 2022. PMID: 35690912 Free PMC article.
-
Tolterodine is a novel candidate for assessing CYP3A4 activity through metabolic volatiles to predict drug responses.Sci Rep. 2025 Jan 20;15(1):2462. doi: 10.1038/s41598-025-86450-9. Sci Rep. 2025. PMID: 39828876 Free PMC article.
-
Meloxicam methyl group determines enzyme specificity for thiazole bioactivation compared to sudoxicam.Toxicol Lett. 2021 Mar 1;338:10-20. doi: 10.1016/j.toxlet.2020.11.015. Epub 2020 Nov 27. Toxicol Lett. 2021. PMID: 33253783 Free PMC article.
References
-
- Omura T (1999) Forty years of cytochrome P450. Biochem Biophys Res Commun 266: 690–698. - PubMed
-
- Franklin MR, Hathaway LB (2008) 2-Diethylaminoethyl-2,2-diphenylvalerate-HCl (SKF-525A) revisited: comparative cytochrome P450 inhibition in human liver microsomes by SKR525A, its metabolites, and SKF-acid and SKF-alcohol. Drug Metab Dispos 36: 2539–2546. - PubMed
-
- Rossi M (1983) Structural studies of metyrapone: A potent inhibitor of cytochrome P-450. J Med Chem 26: 1246–1252. - PubMed
-
- Guengerich FP (2009) Inhibition of drug metabolizing enzymes. Handbook of Drug Metabolism, 2nd Ed. In: Pearson PG, Wienkers LC (eds.) pp: 203–226.
-
- Correia MA, Hollenberg PF (2015) Inhibition of cytochrome P450 enzymes. Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th Ed. In: Ortiz de Montellano PR (ed.) pp: 177–259.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
