The adjuvant GLA-AF enhances human intradermal vaccine responses
- PMID: 30221194
- PMCID: PMC6136895
- DOI: 10.1126/sciadv.aas9930
The adjuvant GLA-AF enhances human intradermal vaccine responses
Abstract
Adjuvants are key to shaping the immune response to vaccination, but to date, no adjuvant suitable for human use has been developed for intradermal vaccines. These vaccines could be self-administered and sent through the mail as they do not require long needles or technical expertise in immunization. In the event of a pandemic outbreak, this approach could alleviate the congregation of patients in health centers and thus reduce the potential of these centers to enhance the spread of lethal infection. A reliable and potent vaccine system for self-administration would provide an effective countermeasure for delivery through existing product distribution infrastructure. We report results from preclinical and clinical trials that demonstrate the feasibility of an adjuvanted, intradermal vaccine that induced single shot protection in ferrets and seroprotection in humans against one of the more lethal strains of pandemic flu, Indonesia H5N1. In the human trial, the vaccine was safe and clinical responses were above approvable endpoints for a protective flu vaccine. Inclusion of a modern TLR4 (Toll-like receptor 4) agonist-based adjuvant was critical to the development of the response in the intradermal groups. In humans, this is the first report of a safe and effective intradermal adjuvant, GLA-AF (aqueous formulation of glucopyranosyl lipid adjuvant), and provides a future path for developing a vaccine-device combination for distribution by mail and self-administration in case of a pandemic.
Figures
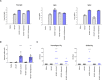

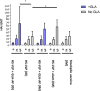
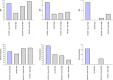
References
-
- Hütter J., Rödig J. V., Höper D., Seeberger P. H., Reichl U., Rapp E., Lepenies B., Toward animal cell culture-based influenza vaccine design: Viral hemagglutinin N-glycosylation markedly impacts immunogenicity. J. Immunol. 190, 220–230 (2013). - PubMed
-
- van Wielink R., Harmsen M. M., Martens D. E., de Leeuw O. S., Peeters B. P. H., Wijffels R. H., Moormann R. J. M., Effect of natural and chimeric haemagglutinin genes on influenza A virus replication in baby hamster kidney cells. J. Biotechnol. 162, 197–201 (2012). - PubMed
-
- Khurana S., Larkin C., Verma S., Joshi M. B., Fontana J. M., Steven A. C., King L. R., Manischewitz J., McCormick W., Gupta R. K., Golding H., Recombinant HA1 produced in E. coli forms functional oligomers and generates strain-specific SRID potency antibodies for pandemic influenza vaccines. Vaccine 29, 5657–5665 (2011). - PMC - PubMed
-
- Baxter R., Patriarca P. A., Ensor K., Izikson R., Goldenthal K. L., Cox M. M., Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50–64 years of age. Vaccine 29, 2272–2278 (2011). - PubMed
-
- D’Aoust M.-A., Lavoie P.-O., Couture M. M.-J., Trépanier S., Guay J.-M., Dargis M., Mongrand S., Landry N., Ward B. J., Vézina L.-P., Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 6, 930–940 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

