Controlled Dye Aggregation in Sodium Dodecylsulfate-Stabilized Poly(methylmethacrylate) Nanoparticles as Fluorescent Imaging Probes
- PMID: 30221237
- PMCID: PMC6130898
- DOI: 10.1021/acsomega.8b00785
Controlled Dye Aggregation in Sodium Dodecylsulfate-Stabilized Poly(methylmethacrylate) Nanoparticles as Fluorescent Imaging Probes
Abstract
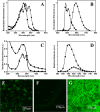
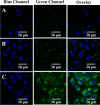
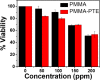
Polymer nanoparticles are used extensively in biomedical applications. Poly(methylmethacrylate) (PMMA) nanoparticles obtained via nanoprecipitation were unstable and flocculate or precipitate from solution within a few hours. A simple method to improve the stability of the particles using surfactants at low concentrations was carried out to produce PMMA nanoparticles with long-term stability in water (>6 months). The increased stability was attributed to the incorporation of surfactants inside the polymer particles during nanoprecipitation. The same methodology was also adopted to encapsulate a highly fluorescent hydrophobic perylene tetraester inside the polymer nanoparticles with good stability in water. Because of the presence of the anionic sodium dodecyl sulfate, the particles showed a negative zeta potential of -34.7 mV and an average size of 150 nm. Similarly, the dye-encapsulated polymer nanoparticles showed a zeta potential of -35.1 mV and an average particle size of 180 nm. By varying the concentration of encapsulated dyes inside the polymer nanoparticles, dye aggregation could be controlled, and the fluorescence profiles of the nanoparticles were altered. To understand the uptake and toxicity of the polymer nanoparticles, baby hamster kidney cells were chosen as a model system. The polymer nanoparticles were taken up by the cells within 3 h and were nontoxic at concentrations as high as 100 ppm. The confocal micrographs of the cells revealed localized fluorescence from the polymer nanoparticles around the nucleus in the cytoplasm without the penetration of the nuclear envelope.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Zhang C.; Pansare V. J.; Prud’homme R. K.; Priestley R. D. Flash nanoprecipitation of polystyrenenanoparticles. Soft Matter 2012, 8, 86–93. 10.1039/c1sm06182h. - DOI
-
- Geissler A.; Biesalski M.; Heinze T.; Zhang K. Formation of nanostructured cellulose stearoyl esters via nanoprecipitation. J. Mater. Chem. A 2014, 2, 1107–1116. 10.1039/c3ta13937a. - DOI
-
- Miao M.; Chen Q.; Zhang C.; Cao X.; Zhou W.; Qiu Q.; An Z. Nanoprecipitation of PMMA Stabilized by Core Cross-Linked Star Polymers. Macromol. Chem. Phys. 2013, 214, 1158–1164. 10.1002/macp.201300234. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

