When nucleoside chemistry met hypervalent iodine reagents
- PMID: 30221252
- PMCID: PMC6135263
- DOI: 10.24820/ark.5550190.p010.281
When nucleoside chemistry met hypervalent iodine reagents
Abstract
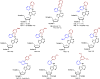
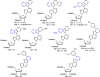
There has been increasing use of hypervalent iodine reagents in the field of nucleoside chemistry. Applications span: (a) synthesis of nucleoside analogues with sulfur and seleno sugar surrogates, (b) synthesis of unusual carbocyclic and ether ring-containing nucleosides, (c) introduction of sulfur and selenium into pyrimidine bases of nucleosides and analogues, (d) synthesis of isoxazole and isoxazoline ring-containing nucleoside analogues, (e) involvement of purine ring nitrogen atoms for remote C-H bond oxidation, and (f) metal-catalyzed and uncatalyzed synthesis of benzimidazolyl purine nucleoside analogues by intramolecular C-N bond formation. This review offers a perspective on developments involving the use of hypervalent iodine reagents in the field of nucleoside chemistry that have appeared in the literature in the 2003-2017 time frame.
Keywords: C-H bond activation; C-N bond formation; hypervalent iodine; nucleoside analogs; nucleosides.
Figures


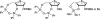





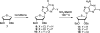
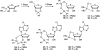
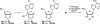
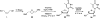
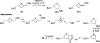
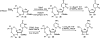



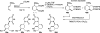
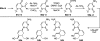


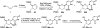










Similar articles
-
Glycosylation reactions mediated by hypervalent iodine: application to the synthesis of nucleosides and carbohydrates.Beilstein J Org Chem. 2018 Jun 28;14:1595-1618. doi: 10.3762/bjoc.14.137. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30013687 Free PMC article. Review.
-
Pd-catalyzed versus uncatalyzed, PhI(OAc)2-mediated cyclization reactions of N6-([1,1'-biaryl]-2-yl)adenine nucleosides.ChemCatChem. 2017 Nov 9;9(21):4058-4069. doi: 10.1002/cctc.201700918. Epub 2017 Oct 24. ChemCatChem. 2017. PMID: 29503672 Free PMC article.
-
Cyclic Hypervalent Iodine Reagents: Enabling Tools for Bond Disconnection via Reactivity Umpolung.Acc Chem Res. 2018 Dec 18;51(12):3212-3225. doi: 10.1021/acs.accounts.8b00468. Epub 2018 Nov 28. Acc Chem Res. 2018. PMID: 30485071
-
Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions.Front Chem. 2020 Sep 29;8:705. doi: 10.3389/fchem.2020.00705. eCollection 2020. Front Chem. 2020. PMID: 33134246 Free PMC article. Review.
-
Palladium-Catalyzed Organic Reactions Involving Hypervalent Iodine Reagents.Molecules. 2022 Jun 17;27(12):3900. doi: 10.3390/molecules27123900. Molecules. 2022. PMID: 35745020 Free PMC article. Review.
References
-
- Herdewijn P, editor. Biochemistry, Biotechnology and Medicine. Wiley-VCH; Weinheim, Germany: 2008. Modified Nucleosides.
-
- Vaghefi M, editor. Nucleoside Triphosphates and Their Analogs: Chemistry, Biotechnology, and Biological Applications. CRC Press; Boca Raton, FL: 2005.
-
- Chu CK, editor. Antiviral Nucleosides: Chiral Synthesis and Chemotherapy. Elsevier; Amsterdam: 2003.
-
- Jordheim LP, Durantel D, Zoulim F, Dumontet C. Nature Rev Drug Discov. 2013;12:447–464. - PubMed
-
- Damaraju VL, Damaraju S, Young JD, Baldwin SA, Mackey J, Sawyer MB, Cass CE. Oncogene. 2003;22:7524–7536. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
