Chiral Aniline Synthesis via Stereospecific C(sp3)-C(sp2) Coupling of Boronic Esters with Aryl Hydrazines
- PMID: 30221524
- PMCID: PMC6178205
- DOI: 10.1021/acs.orglett.8b02615
Chiral Aniline Synthesis via Stereospecific C(sp3)-C(sp2) Coupling of Boronic Esters with Aryl Hydrazines
Abstract
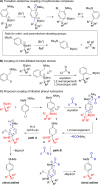
An enantiospecific coupling between alkylboronic esters and lithiated aryl hydrazines is described. The reaction proceeds under transition-metal-free conditions and is promoted by acylation of a hydrazinyl arylboronate complex, which triggers a N-N bond cleavage with concomitant 1,2-metalate rearrangement. Judicious choice of the acylating agent enabled the synthesis of ortho- and para-substituted anilines with essentially complete enantiospecificity from a wide range of boronic ester substrates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

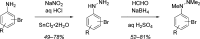



Similar articles
-
Enantiospecific Synthesis of ortho-Substituted Benzylic Boronic Esters by a 1,2-Metalate Rearrangement/1,3-Borotropic Shift Sequence.J Am Chem Soc. 2017 Jul 19;139(28):9519-9522. doi: 10.1021/jacs.7b05880. Epub 2017 Jul 6. J Am Chem Soc. 2017. PMID: 28661133 Free PMC article.
-
Chiral Benzothiophene Synthesis via Enantiospecific Coupling of Benzothiophene S-Oxides with Boronic Esters.Angew Chem Int Ed Engl. 2021 Nov 22;60(48):25313-25317. doi: 10.1002/anie.202112180. Epub 2021 Nov 2. Angew Chem Int Ed Engl. 2021. PMID: 34582085
-
Enantiospecific Synthesis of ortho-Substituted 1,1-Diarylalkanes by a 1,2-Metalate Rearrangement/anti-SN 2' Elimination/Rearomatizing Allylic Suzuki-Miyaura Reaction Sequence.Angew Chem Int Ed Engl. 2019 Jan 28;58(5):1366-1370. doi: 10.1002/anie.201811343. Epub 2018 Dec 21. Angew Chem Int Ed Engl. 2019. PMID: 30520228 Free PMC article.
-
Homologation and alkylation of boronic esters and boranes by 1,2-metallate rearrangement of boronate complexes.Chem Rec. 2009;9(1):24-39. doi: 10.1002/tcr.20168. Chem Rec. 2009. PMID: 19243084 Review.
-
Transition Metal Catalyst Free Synthesis of Olefins from Organoboron Derivatives.Chemistry. 2022 Apr 19;28(22):e202104125. doi: 10.1002/chem.202104125. Epub 2022 Feb 28. Chemistry. 2022. PMID: 35137987 Free PMC article. Review.
Cited by
-
Atom and step economical synthesis of acyclic quaternary centers via iridium-catalyzed hydroarylative cross-coupling of 1,1-disubstituted alkenes.Chem Sci. 2022 Sep 13;13(37):11183-11189. doi: 10.1039/d2sc02790a. eCollection 2022 Sep 28. Chem Sci. 2022. PMID: 36320466 Free PMC article.
-
Stereospecific 1,2-Migrations of Boronate Complexes Induced by Electrophiles.Angew Chem Int Ed Engl. 2020 Sep 21;59(39):16859-16872. doi: 10.1002/anie.202008096. Epub 2020 Jul 22. Angew Chem Int Ed Engl. 2020. PMID: 32592274 Free PMC article. Review.
References
-
- Mehellou Y.; De Clercq E. J. Med. Chem. 2010, 53, 521–538. 10.1021/jm900492g. - DOI - PubMed
- Fürst S.; Friedmann T.; Bartolini A.; Bartolini R.; Aiello-Malmberg P.; Galli A.; Somogyi G. T.; Knoll J. Eur. J. Pharmacol. 1982, 83, 233–241. 10.1016/0014-2999(82)90256-4. - DOI - PubMed
- Hödl C.; Raunegger K.; Strommer R.; Ecker G. F.; Kunert O.; Sturm S.; Seger C.; Haslinger E.; Steiner R.; Strauss W. S. L.; Schramm H.-W. J. Med. Chem. 2009, 52, 1268–1274. 10.1021/jm800985z. - DOI - PubMed
- Chung C. K.; Bulger P. G.; Kosjek B.; Belyk K. M.; Rivera N.; Scott M. E.; Humphrey G. R.; Limanto J.; Bachert D. C.; Emerson K. M. Org. Process Res. Dev. 2014, 18, 215–227. 10.1021/op400233z. - DOI
-
- Leonori D.; Aggarwal V. K. Angew. Chem., Int. Ed. 2015, 54, 1082–1096. 10.1002/anie.201407701. - DOI - PubMed
- Rygus J. P. G.; Crudden C. M. J. Am. Chem. Soc. 2017, 139, 18124–18137. 10.1021/jacs.7b08326. - DOI - PubMed
- Wang C.-Y.; Derosa J.; Biscoe M. R. Chem. Sci. 2015, 6, 5105–5113. 10.1039/C5SC01710F. - DOI - PMC - PubMed
- Cherney A. H.; Kadunce N. T.; Reisman S. E. Chem. Rev. 2015, 115, 9587–9652. 10.1021/acs.chemrev.5b00162. - DOI - PMC - PubMed
-
-
For stereospecific cross-couplings of alkylboronic esters, see:
- Zhou S.-M.; Deng M.-Z.; Xia L.-J.; Tang M.-H. Angew. Chem., Int. Ed. 1998, 37, 2845–2847. 10.1002/(SICI)1521-3773(19981102)37:20<2845::AID-ANIE2845>3.0.CO;2-U. - DOI - PubMed
- Imao D.; Glasspoole B. W.; Laberge V. S.; Crudden C. M. J. Am. Chem. Soc. 2009, 131, 5024–5025. 10.1021/ja8094075. - DOI - PubMed
- Ohmura T.; Awano T.; Suginome M. J. Am. Chem. Soc. 2010, 132, 13191–13193. 10.1021/ja106632j. - DOI - PubMed
- Awano T.; Ohmura T.; Suginome M. J. Am. Chem. Soc. 2011, 133, 20738–20741. 10.1021/ja210025q. - DOI - PubMed
- Glasspoole B. W.; Oderinde M. S.; Moore B. D.; Antoft-Finch A.; Crudden C. M. Synthesis 2013, 45, 1759–1763. 10.1055/s-0033-1338875. - DOI
- Matthew S. C.; Glasspoole B. W.; Eisenberger P.; Crudden C. M. J. Am. Chem. Soc. 2014, 136, 5828–5831. 10.1021/ja412159g. - DOI - PubMed
- Blaisdell T. P.; Morken J. P. J. Am. Chem. Soc. 2015, 137, 8712–8715. 10.1021/jacs.5b05477. - DOI - PMC - PubMed
- Crudden C. M.; Ziebenhaus C.; Rygus J. P. G.; Ghozati K.; Unsworth P. J.; Nambo M.; Voth S.; Hutchinson M.; Laberge V. S.; Maekawa Y.; Imao D. Nat. Commun. 2016, 7, 11065.10.1038/ncomms11065. - DOI - PMC - PubMed
-
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

