Immune-mediated Cerebellar Ataxias: Practical Guidelines and Therapeutic Challenges
- PMID: 30221603
- PMCID: PMC6341499
- DOI: 10.2174/1570159X16666180917105033
Immune-mediated Cerebellar Ataxias: Practical Guidelines and Therapeutic Challenges
Abstract
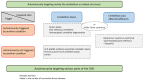
Immune-mediated cerebellar ataxias (IMCAs), a clinical entity reported for the first time in the 1980s, include gluten ataxia (GA), paraneoplastic cerebellar degenerations (PCDs), antiglutamate decarboxylase 65 (GAD) antibody-associated cerebellar ataxia, post-infectious cerebellitis, and opsoclonus myoclonus syndrome (OMS). These IMCAs share common features with regard to therapeutic approaches. When certain factors trigger immune processes, elimination of the antigen( s) becomes a priority: e.g., gluten-free diet in GA and surgical excision of the primary tumor in PCDs. Furthermore, various immunotherapeutic modalities (e.g., steroids, immunoglobulins, plasmapheresis, immunosuppressants, rituximab) should be considered alone or in combination to prevent the progression of the IMCAs. There is no evidence of significant differences in terms of response and prognosis among the various types of immunotherapies. Treatment introduced at an early stage, when CAs or cerebellar atrophy is mild, is associated with better prognosis. Preservation of the "cerebellar reserve" is necessary for the improvement of CAs and resilience of the cerebellar networks. In this regard, we emphasize the therapeutic principle of "Time is Cerebellum" in IMCAs.
Keywords: Cerebellar ataxias; anti-GAD65Ab-associated cerebellar ataxia; gluten ataxia; immune-mediated cerebellar ataxias; immunotherapy; opsoclonus myoclonus syndrome; paraneoplastic cerebellar degeneration; post-infectious cerebellitis; prognosis; therapy; treatment..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures


References
-
- Hadjivassiliou M. Immune-mediated acquired ataxias. Handb. Clin. Neurol. 2012;103:189–199. [http://dx.doi.org/10.1016/B978-0-444-51892-7.00011-5]. [PMID: 21827889]. - PubMed
-
- Hadjivassiliou M., Grünewald R.A., Chattopadhyay A.K., Davies-Jones G.A., Gibson A., Jarratt J.A., Kandler R.H., Lobo A., Powell T., Smith C.M. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352(9140):1582–1585. [http://dx.doi.org/10.1016/S0140-6736(98)05342-2]. [PMID: 9843103]. - PubMed
-
- Graus F., Delattre J.Y., Antoine J.C., Dalmau J., Giometto B., Grisold W., Honnorat J., Smitt P.S., Vedeler Ch., Verschuuren J.J., Vincent A., Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1135–1140. [http://dx.doi.org/10.1136/jnnp. 2003.034447]. [PMID: 15258215]. - PMC - PubMed
-
- Dalmau J., Rosenfeld M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327–340. [http://dx.doi.org/10.1016/ S1474-4422(08)70060-7]. [PMID: 18339348]. - PMC - PubMed
-
- Ducray F., Demarquay G., Graus F., Decullier E., Antoine J.C., Giometto B., Psimaras D., Delattre J.Y., Carpentier A.F., Honnorat J. Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur. J. Neurol. 2014;21(5):731–735. [http://dx.doi.org/10.1111/ene.12368]. [PMID: 24471811]. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

