FKBP11 protects intestinal epithelial cells against inflammation‑induced apoptosis via the JNK‑caspase pathway in Crohn's disease
- PMID: 30221722
- PMCID: PMC6172375
- DOI: 10.3892/mmr.2018.9485
FKBP11 protects intestinal epithelial cells against inflammation‑induced apoptosis via the JNK‑caspase pathway in Crohn's disease
Erratum in
-
[Corrigendum] FKBP11 protects intestinal epithelial cells against inflammation‑induced apoptosis via the JNK‑caspase pathway in Crohn's disease.Mol Med Rep. 2024 Mar;29(3):50. doi: 10.3892/mmr.2024.13172. Epub 2024 Feb 1. Mol Med Rep. 2024. PMID: 38299256 Free PMC article.
Abstract
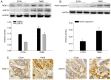
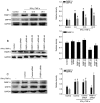
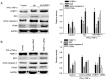
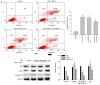
Endoplasmic reticulum (ER) stress in intestinal epithelial cells (IECs) has an important role in the pathogenesis of Crohn's disease (CD). FK506 binding protein 11 (FKBP11), a member of the peptidyl‑prolyl cis‑trans isomerase family, is involved in the unfolded protein response (UPR) and is closely associated with inflammation. Previous bioinformatics analysis revealed a potential association between FKBP11 and human CD. Thus, the present study aimed to investigate the potential significance of FKBP11 in IEC homeostasis and CD. In the present study, increased expression of FKBP11 was detected in the intestinal inflammatory tissues of patients with CD. Furthermore, the results of the present study revealed that overexpression of FKBP11 was accompanied by increased expression levels of the ER stress marker 78 kDa glucose‑regulated protein in the colon tissues of a 2, 4, 6‑trinitrobenzenesulphonic acid‑induced mouse colitis model. Using interferon‑γ (IFN‑γ)/tumor necrosis factor‑α (TNF‑α)‑stimulated IECs as an ER stress and apoptosis cell model, the associated of FKBP11 with ER stress and apoptosis levels was confirmed in IECs. Overexpression of FKBP11 was revealed to significantly attenuate the elevated expression of pro‑apoptotic proteins (Bcl2 associated X apoptosis regulator, caspase‑12 and active caspase‑3), suppress the phosphorylation of c‑Jun N‑terminal kinase (JNK), and decrease apoptosis of IFN‑γ/TNF‑α stimulated IECs. Knockdown of FKBP11 by transfection with small interfering RNA further validated the aforementioned results. In conclusion, these results suggest that the UPR protein FKBP11 may protect IECs against IFN‑γ/TNF‑α induced apoptosis by inhibiting the ER stress‑associated JNK/caspase apoptotic pathway in CD.
Figures


References
-
- Cheng L, Huang MF, Mei PF, Bo WH, Deng CS. The clinical, endoscopic and pathologic features of Crohn's disease in the differentiation from intestinal tuberculosis. Zhonghua Nei Ke Za Zhi. 2013;52:940–944. (In Chinese) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

