Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation
- PMID: 30221728
- PMCID: PMC6172393
- DOI: 10.3892/mmr.2018.9488
Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation
Abstract
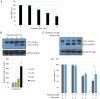
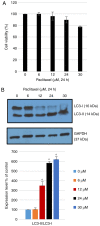
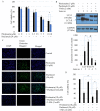
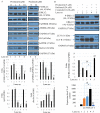
Pristimerin, a quinonemethide triterpenoid, has demonstrated anticancer activity against a number of types of cancer, including breast cancer. However, its mechanism of action remains unclear. The present study investigated the autophagy‑induced anticancer efficacy of pristimerin on MDA‑MB‑231 human breast cancer cells. Pristimerin inhibited the growth of these cells in a concentration‑dependent manner. Treatment with pristimerin dose‑dependently induced an increase of light chain 3B (LC3‑II), whereas autophagy inhibitor 3‑methyladenine (3‑MA) inhibited pristimerin‑induced LC3‑II accumulation and cytotoxic effects. Autophagy was also activated by paclitaxel as observed by an elevated LC3‑II level. Although 24 µM paclitaxel induced autophagy without cytotoxicity, combined with pristimerin it additively induced cell growth inhibition and autophagy induction. Autophagy induction was measured with an autophagy detection kit and LC3‑II levels were monitored by western blot analysis. Treatment with 3‑MA inhibited LC3‑II accumulation and cell death induced by a combination of paclitaxel and pristimerin. Pristimerin and paclitaxel inhibited extracellular signal‑regulated kinase (ERK)1/2/p90RSK signaling, consistent with autophagy indicators, namely p62 degradation and beclin 1 expression. In addition, ERK activator ceramide C6 treatment suppressed the LC3‑II levels induced by a combination of paclitaxel and pristimerin. These results suggested that exposure to pristimerin induced autophagic cell death, whereas a combination treatment of pristimerin and paclitaxel resulted in an additive effect on ERK‑dependent autophagic cell death.
Figures
Similar articles
-
Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity.Int J Cancer. 2005 May 20;115(1):19-35. doi: 10.1002/ijc.20754. Int J Cancer. 2005. PMID: 15657900
-
Anticancer activity of pristimerin in epidermal growth factor receptor 2-positive SKBR3 human breast cancer cells.Biol Pharm Bull. 2013;36(2):316-25. doi: 10.1248/bpb.b12-00685. Biol Pharm Bull. 2013. PMID: 23370361
-
Pristimerin Promotes Ubiquitination of HSPA8 and Activates the VAV1/ERK Pathway to Suppress TNBC Proliferation.Adv Sci (Weinh). 2025 Mar;12(10):e2413174. doi: 10.1002/advs.202413174. Epub 2025 Jan 15. Adv Sci (Weinh). 2025. PMID: 39813169 Free PMC article.
-
Pristimerin Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review.Drug Des Devel Ther. 2024 May 18;18:1673-1694. doi: 10.2147/DDDT.S460093. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38779590 Free PMC article. Review.
-
Anticancer Potential and Molecular Targets of Pristimerin: A Mini- Review.Curr Cancer Drug Targets. 2017;17(2):100-108. doi: 10.2174/1568009616666160112105824. Curr Cancer Drug Targets. 2017. PMID: 26758533 Review.
Cited by
-
Novel Anti-Cancer Products Targeting AMPK: Natural Herbal Medicine against Breast Cancer.Molecules. 2023 Jan 11;28(2):740. doi: 10.3390/molecules28020740. Molecules. 2023. PMID: 36677797 Free PMC article. Review.
-
Pristimerin, a triterpene that inhibits monoacylglycerol lipase activity, prevents the development of paclitaxel-induced allodynia in mice.Front Pharmacol. 2022 Aug 9;13:944502. doi: 10.3389/fphar.2022.944502. eCollection 2022. Front Pharmacol. 2022. PMID: 36016571 Free PMC article.
-
Research Advances in Clinical Applications, Anticancer Mechanism, Total Chemical Synthesis, Semi-Synthesis and Biosynthesis of Paclitaxel.Molecules. 2023 Nov 10;28(22):7517. doi: 10.3390/molecules28227517. Molecules. 2023. PMID: 38005238 Free PMC article. Review.
-
Autophagy modulating agents as chemosensitizers for cisplatin therapy in cancer.Invest New Drugs. 2021 Apr;39(2):538-563. doi: 10.1007/s10637-020-01032-y. Epub 2020 Nov 7. Invest New Drugs. 2021. PMID: 33159673 Free PMC article. Review.
-
Accurate delivery of pristimerin and paclitaxel by folic acid-linked nano-micelles for enhancing chemosensitivity in cancer therapy.Nano Converg. 2022 Nov 24;9(1):52. doi: 10.1186/s40580-022-00343-5. Nano Converg. 2022. PMID: 36427092 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

