Disulfide reductase systems in liver
- PMID: 30221761
- PMCID: PMC6346074
- DOI: 10.1111/bph.14498
Disulfide reductase systems in liver
Abstract
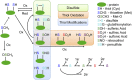
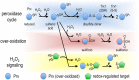
Intermediary metabolism and detoxification place high demands on the disulfide reductase systems in most hepatocyte subcellular compartments. Biosynthetic, metabolic, cytoprotective and signalling activities in the cytosol; regulation of transcription in nuclei; respiration in mitochondria; and protein folding in endoplasmic reticulum all require resident disulfide reductase activities. In the cytosol, two NADPH-dependent enzymes, glutathione reductase and thioredoxin reductase, as well as a recently identified NADPH-independent system that uses catabolism of methionine to maintain pools of reduced glutathione, supply disulfide reducing power. However the necessary discontinuity between the cytosol and the interior of organelles restricts the ability of the cytosolic systems to support needs in other compartments. Maintenance of molecular- and charge-gradients across the inner-mitochondrial membrane, which is needed for oxidative phosphorylation, mandates that the matrix maintain an autonomous set of NADPH-dependent disulfide reductase systems. Elsewhere, complex mechanisms mediate the transfer of cytosolic reducing power into specific compartments. The redox needs in each compartment also differ, with the lumen of the endoplasmic reticulum, the mitochondrial inter-membrane space and some signalling proteins in the cytosol each requiring different levels of protein oxidation. Here, we present an overview of the current understanding of the disulfide reductase systems in major subcellular compartments of hepatocytes, integrating knowledge obtained from direct analyses on liver with inferences from other model systems. Additionally, we discuss relevant advances in the expanding field of redox signalling. LINKED ARTICLES: This article is part of a themed section on Chemical Biology of Reactive Sulfur Species. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.4/issuetoc.
© 2018 The British Pharmacological Society.
Figures


References
-
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP (2011). In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab 14: 819–829. - PubMed
-
- Arner ES, Holmgren A (2000). Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109. - PubMed
-
- Arnér ESJ (2009). Focus on mammalian thioredoxin reductases – important selenoproteins with versatile functions. Biochim Biophys Acta 1790: 495–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

