Ceftazidime-Avibactam Population Pharmacokinetic Modeling and Pharmacodynamic Target Attainment Across Adult Indications and Patient Subgroups
- PMID: 30221827
- PMCID: PMC6440567
- DOI: 10.1111/cts.12585
Ceftazidime-Avibactam Population Pharmacokinetic Modeling and Pharmacodynamic Target Attainment Across Adult Indications and Patient Subgroups
Abstract
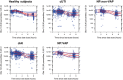
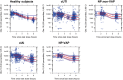
Ceftazidime-avibactam is a novel β-lactam/β-lactamase inhibitor combination for the treatment of serious infections caused by resistant gram-negative pathogens. Population pharmacokinetic (PopPK) models were built to incorporate pharmacokinetic (PK) data from five phase III trials in patients with complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI), or nosocomial (including ventilator-associated) pneumonia. Ceftazidime and avibactam pharmacokinetics were well-described by two-compartment disposition models, with creatinine clearance (CrCL) the key covariate determining clearance variability. Steady-state ceftazidime and avibactam exposure for most patient subgroups differed by ≤ 20% vs. healthy volunteers. Probability of PK/pharmacodynamic (PD) target attainment (free plasma ceftazidime > 8 mg/L and avibactam > 1 mg/L for ≥ 50% of dosing interval) was ≥ 94.9% in simulations for all patient subgroups, including indication and renal function categories. No exposure-microbiological response relationship was identified because target exposures were achieved in almost all patients. These modeling results support the approved ceftazidime-avibactam dosage regimens (2000-500 mg every 8 hours, adjusted for CrCL ≤ 50 mL/min).
Trial registration: ClinicalTrials.gov NCT01291602 NCT01430910 NCT01920399 NCT01499290 NCT01500239 NCT01726023 NCT01644643 NCT01595438 NCT01599806 NCT01808092 NCT01624246.
© 2018 University of Liverpool. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Jianguo Li and Shampa Das are former employees of and shareholders in AstraZeneca. Diansong Zhou is an employee of and shareholder in AstraZeneca. Mark Lovern, Michelle Green, Craig Comisar, and Yuan Xiong are employees of Certara Strategic Consulting (formerly Quantitative Solutions), and James Wright is an employee of Wright Dose Ltd, both of which received funding from AstraZeneca for support and assistance with the PopPK analyses. Merran MacPherson is a former employee of Wright Dose Ltd. and also holds shares in AstraZeneca. Joannellyn Chiu and Jeremy Hing are former employees of Certara Strategic Consulting. Todd Riccobene and Timothy J. Carrothers are employees of and shareholders in Allergan (formerly Actavis, formerly Forest Laboratories).
Figures

References
-
- Barbier, F. , Andremont, A. , Wolff, M. & Bouadma, L. Hospital‐acquired pneumonia and ventilator‐associated pneumonia: recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 19, 216–228 (2013). - PubMed
-
- Nicolle, L.E. Urinary tract infection. Crit. Care Clin. 29, 699–715 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

