Synergistic Targeting HER2 and EGFR with Bivalent Aptamer-siRNA Chimera Efficiently Inhibits HER2-Positive Tumor Growth
- PMID: 30222359
- PMCID: PMC6220360
- DOI: 10.1021/acs.molpharmaceut.8b00388
Synergistic Targeting HER2 and EGFR with Bivalent Aptamer-siRNA Chimera Efficiently Inhibits HER2-Positive Tumor Growth
Abstract
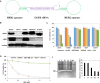
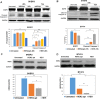
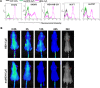
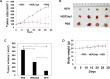
HER2 overexpression is identified on 20-30% breast cancer and other cancers at different levels. Although HER2 targeted monoclonal antibody combined with chemical drugs has shown improved outcomes in HER2 expressing patients, drug resistance and toxicity have limited their efficacy. To overcome drug resistance, cotargeting multiple HER receptors was proven to be effective. EGFR/HER2 dimerization can active PI3K/AKT pathway, and resistance to HER2-targeted drugs is associated with upregulation of EGFR. Here, we developed a novel HER2/EGFR targeted nucleic acid therapeutic to address current drug limits. The new therapeutic is constructed by fusing HER2 aptamer-EGFR siRNA sense strand with HER2 aptamer-EGFR siRNA antisense strand into one molecule: a bivalent HER2 aptamer-EGFR siRNA aptamer chimera (HEH). In breast cancer cell lines, HEH can be selectively taken up into HER2 expressing cells and successfully silence EGFR gene and down regulate HER2 expression. In breast cancer xenograft models, HEH is capable of triggering cell apoptosis, decreasing HER2 and EGFR expression, and suppressing tumor growth. The therapeutic efficacy of HEH is superior to HER2 aptamer only, which suggests that HEH has synergistic effect by targeting HER2 and EGFR. This study demonstrated that HEH has great potential as a new HER2 targeted drug to address toxicity and resistance of current drugs and may provide a cure for many HER2 positive cancers.
Keywords: HER2; aptamer; bivalent; siRNA; siRNA delivery; synergistic treatment; targeted therapeutic.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Targeting EGFR/HER2/HER3 with a Three-in-One Aptamer-siRNA Chimera Confers Superior Activity against HER2+ Breast Cancer.Mol Ther Nucleic Acids. 2018 Mar 2;10:317-330. doi: 10.1016/j.omtn.2017.12.015. Epub 2017 Dec 30. Mol Ther Nucleic Acids. 2018. PMID: 29499944 Free PMC article.
-
Aptamer-functionalized hybrid nanoparticle for the treatment of breast cancer.Eur J Pharm Biopharm. 2017 May;114:108-118. doi: 10.1016/j.ejpb.2017.01.011. Epub 2017 Jan 25. Eur J Pharm Biopharm. 2017. PMID: 28131717 Free PMC article.
-
Development of HER2-Specific Aptamer-Drug Conjugate for Breast Cancer Therapy.Int J Mol Sci. 2020 Dec 21;21(24):9764. doi: 10.3390/ijms21249764. Int J Mol Sci. 2020. PMID: 33371333 Free PMC article.
-
Irreversible multitargeted ErbB family inhibitors for therapy of lung and breast cancer.Curr Cancer Drug Targets. 2015;14(9):775-93. doi: 10.2174/1568009614666141111104643. Curr Cancer Drug Targets. 2015. PMID: 25435079 Review.
-
Aptamer-siRNA chimeras: Promising tools for targeting HER2 signaling in cancer.Chem Biol Drug Des. 2023 May;101(5):1162-1180. doi: 10.1111/cbdd.14143. Epub 2022 Sep 25. Chem Biol Drug Des. 2023. PMID: 36099164 Review.
Cited by
-
DNA-Based Nanomaterials as Drug Delivery Platforms for Increasing the Effect of Drugs in Tumors.Cancers (Basel). 2023 Apr 5;15(7):2151. doi: 10.3390/cancers15072151. Cancers (Basel). 2023. PMID: 37046816 Free PMC article. Review.
-
Anti-HER2 therapy in metastatic breast cancer: many choices and future directions.Cancer Metastasis Rev. 2022 Mar;41(1):193-209. doi: 10.1007/s10555-022-10021-x. Epub 2022 Feb 10. Cancer Metastasis Rev. 2022. PMID: 35142964 Free PMC article. Review.
-
Aptamers as Delivery Agents of siRNA and Chimeric Formulations for the Treatment of Cancer.Pharmaceutics. 2019 Dec 16;11(12):684. doi: 10.3390/pharmaceutics11120684. Pharmaceutics. 2019. PMID: 31888119 Free PMC article. Review.
-
Drug Release from Gelsolin-Targeted Phase-Transition Nanoparticles Triggered by Low-Intensity Focused Ultrasound.Int J Nanomedicine. 2022 Jan 6;17:61-71. doi: 10.2147/IJN.S341421. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35023919 Free PMC article.
-
A novel bispecific aptamer targeting LAG3 and HER2 enhances T cell-mediated immunotherapy against HER2-positive cancer cells.Front Immunol. 2025 Jul 21;16:1557910. doi: 10.3389/fimmu.2025.1557910. eCollection 2025. Front Immunol. 2025. PMID: 40761795 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

