Retinal Vasculature in Development and Diseases
- PMID: 30222533
- PMCID: PMC6326083
- DOI: 10.1146/annurev-vision-091517-034018
Retinal Vasculature in Development and Diseases
Retraction in
-
Notice of Withdrawal: Retinal Vasculature in Development and Diseases.Annu Rev Vis Sci. 2020 Oct 15;0:10.1146/annurev-vs-04-091720-200001. doi: 10.1146/annurev-vs-04-091720-200001. Annu Rev Vis Sci. 2020. PMID: 33058721 Free PMC article.
Abstract
The retina is one of the most metabolically active tissues in the body, consuming high levels of oxygen and nutrients. A well-organized ocular vascular system adapts to meet the metabolic requirements of the retina to ensure visual function. Pathological conditions affect growth of the blood vessels in the eye. Understanding the neuronal biological processes that govern retinal vascular development is of interest for translational researchers and clinicians to develop preventive and interventional therapeutics for vascular eye diseases that address early drivers of abnormal vascular growth. This review summarizes the current knowledge of the cellular and molecular processes governing both physiological and pathological retinal vascular development, which is dependent on the interaction among retinal cell populations, including neurons, glia, immune cells, and vascular endothelial cells. We also review animal models currently used for studying retinal vascular development.
Keywords: AMD; DR; ROP; animal model; development; retina; vasculature.
Figures
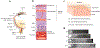
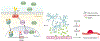
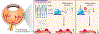

References
-
- Ahmed K, Tunaru S, Tang C, Müller M, Gille A, et al. 2010. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. CellMetab. 11:311–19 - PubMed
-
- Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. 2007. Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Investig. Ophthalmol Vis. Sci. 48:4351–59 - PubMed
-
- Anand-Apte B, Hollyfield JG. 2011. Developmental anatomy of the retinal and choroidal vasculature In The Retina and Its Disorders, ed. Besharse J, pp. 9–15. Oxford, UK: Academic
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

