The physiological variability of channel density in hippocampal CA1 pyramidal cells and interneurons explored using a unified data-driven modeling workflow
- PMID: 30222740
- PMCID: PMC6160220
- DOI: 10.1371/journal.pcbi.1006423
The physiological variability of channel density in hippocampal CA1 pyramidal cells and interneurons explored using a unified data-driven modeling workflow
Abstract
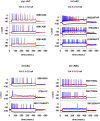
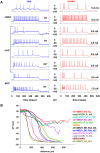
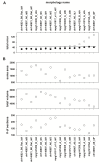
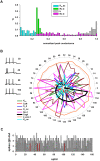
Every neuron is part of a network, exerting its function by transforming multiple spatiotemporal synaptic input patterns into a single spiking output. This function is specified by the particular shape and passive electrical properties of the neuronal membrane, and the composition and spatial distribution of ion channels across its processes. For a variety of physiological or pathological reasons, the intrinsic input/output function may change during a neuron's lifetime. This process results in high variability in the peak specific conductance of ion channels in individual neurons. The mechanisms responsible for this variability are not well understood, although there are clear indications from experiments and modeling that degeneracy and correlation among multiple channels may be involved. Here, we studied this issue in biophysical models of hippocampal CA1 pyramidal neurons and interneurons. Using a unified data-driven simulation workflow and starting from a set of experimental recordings and morphological reconstructions obtained from rats, we built and analyzed several ensembles of morphologically and biophysically accurate single cell models with intrinsic electrophysiological properties consistent with experimental findings. The results suggest that the set of conductances expressed in any given hippocampal neuron may be considered as belonging to two groups: one subset is responsible for the major characteristics of the firing behavior in each population and the other is responsible for a robust degeneracy. Analysis of the model neurons suggests several experimentally testable predictions related to the combination and relative proportion of the different conductances that should be expressed on the membrane of different types of neurons for them to fulfill their role in the hippocampus circuitry.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Mathematical generation of data-driven hippocampal CA1 pyramidal neurons and interneurons copies via A-GLIF models for large-scale networks covering the experimental variability range.Math Biosci. 2024 May;371:109179. doi: 10.1016/j.mbs.2024.109179. Epub 2024 Mar 21. Math Biosci. 2024. PMID: 38521453
-
Computational simulation of the input-output relationship in hippocampal pyramidal cells.J Comput Neurosci. 2006 Oct;21(2):191-209. doi: 10.1007/s10827-006-8797-z. Epub 2006 Jul 25. J Comput Neurosci. 2006. PMID: 16871350
-
Intrinsic and synaptic mechanisms determining the timing of neuron population activity during hippocampal theta oscillation.J Neurophysiol. 2006 Dec;96(6):2889-904. doi: 10.1152/jn.01233.2005. Epub 2006 Aug 9. J Neurophysiol. 2006. PMID: 16899632
-
Analysis and comparison of morphological reconstructions of hippocampal field CA1 pyramidal cells.Hippocampus. 2005;15(3):302-15. doi: 10.1002/hipo.20051. Hippocampus. 2005. PMID: 15490464 Review.
-
The Brain's Best Kept Secret Is Its Degenerate Structure.J Neurosci. 2024 Oct 2;44(40):e1339242024. doi: 10.1523/JNEUROSCI.1339-24.2024. J Neurosci. 2024. PMID: 39358027 Free PMC article. Review.
Cited by
-
Ion-channel regulation of response decorrelation in a heterogeneous multi-scale model of the dentate gyrus.Curr Res Neurobiol. 2021 Mar 5;2:100007. doi: 10.1016/j.crneur.2021.100007. eCollection 2021. Curr Res Neurobiol. 2021. PMID: 33997798 Free PMC article.
-
Introducing the Dendrify framework for incorporating dendrites to spiking neural networks.Nat Commun. 2023 Jan 10;14(1):131. doi: 10.1038/s41467-022-35747-8. Nat Commun. 2023. PMID: 36627284 Free PMC article.
-
Quantitative analysis of the optogenetic excitability of CA1 neurons.Front Comput Neurosci. 2023 Aug 15;17:1229715. doi: 10.3389/fncom.2023.1229715. eCollection 2023. Front Comput Neurosci. 2023. PMID: 37649730 Free PMC article.
-
Degeneracy in hippocampal physiology and plasticity.Hippocampus. 2019 Oct;29(10):980-1022. doi: 10.1002/hipo.23139. Epub 2019 Jul 13. Hippocampus. 2019. PMID: 31301166 Free PMC article. Review.
-
Altered synaptic plasticity at hippocampal CA1-CA3 synapses in Alzheimer's disease: integration of amyloid precursor protein intracellular domain and amyloid beta effects into computational models.Front Comput Neurosci. 2023 Dec 7;17:1305169. doi: 10.3389/fncom.2023.1305169. eCollection 2023. Front Comput Neurosci. 2023. PMID: 38130706 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

