Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves
- PMID: 30222757
- PMCID: PMC6141078
- DOI: 10.1371/journal.pone.0203612
Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves
Abstract
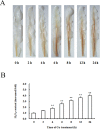
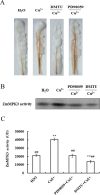
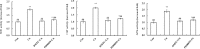
Copper (Cu) is a necessary trace element participated in many physiological processes in plants. But excessive Cu2+ is toxic, which can activate intracellular signals that lead to cellular damage. The mitogen-activated protein kinase (MAPK) cascade is at the center of cell signal transduction and has been reported to be involved in stress-related signaling pathways. ZmMPK3, a kind of MAPKs in maize cells, can be activated by diverse abiotic stresses. In the present study, we investigated the effects of Cu2+ on hydrogen peroxide (H2O2) level, ZmMPK3 activity as well as the activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and ascorbic acid peroxidase (APX) using maize leaf as an experimental model. The results demonstrated that acute Cu2+ exposure for 24 hours led to rapid increases of H2O2 level and the increase in ZmMPK3 activity as well as the total activities of antioxidant enzymes SOD, CAT and APX. H2O2 scavenger, dimethylthiourea (DMTU), effectively inhibited the Cu2+-increased H2O2 level and the activity of ZmMPK3 as well as the activities of the antioxidant enzymes SOD, CAT and APX. Pre-treatment with the MAPK inhibitor, PD98059, significantly blocked the Cu2+-increased activities of ZmMPK3, CAT, APX and SOD, but didn't affect the accumulation of H2O2. Our results suggest that Cu2+ causes oxidative stress to the maize leaves which then activates defense antioxidant enzymes via MAPK pathway. Thus, the signaling pathway is Cu2+-H2O2-ZmMPK3-antioxidant enzymes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

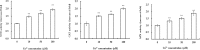
Similar articles
-
Induction of protection against paraquat-induced oxidative damage by abscisic acid in maize leaves is mediated through mitogen-activated protein kinase.J Integr Plant Biol. 2009 Oct;51(10):961-72. doi: 10.1111/j.1744-7909.2009.00868.x. J Integr Plant Biol. 2009. PMID: 19778406
-
Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves.Planta. 2005 Dec;223(1):57-68. doi: 10.1007/s00425-005-0068-0. Epub 2005 Jul 28. Planta. 2005. PMID: 16049674
-
ABA Affects Brassinosteroid-Induced Antioxidant Defense via ZmMAP65-1a in Maize Plants.Plant Cell Physiol. 2015 Jul;56(7):1442-55. doi: 10.1093/pcp/pcv061. Epub 2015 May 4. Plant Cell Physiol. 2015. PMID: 25941233
-
Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses.Int J Mol Sci. 2015 Jun 12;16(6):13561-78. doi: 10.3390/ijms160613561. Int J Mol Sci. 2015. PMID: 26075872 Free PMC article. Review.
-
[Expression regulation of plant ascorbate peroxidase and its tolerance to abiotic stresses].Yi Chuan. 2013 Jan;35(1):45-54. doi: 10.3724/sp.j.1005.2013.00045. Yi Chuan. 2013. PMID: 23357264 Review. Chinese.
Cited by
-
Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China.Environ Sci Pollut Res Int. 2020 Feb;27(5):5211-5221. doi: 10.1007/s11356-019-07264-7. Epub 2019 Dec 17. Environ Sci Pollut Res Int. 2020. PMID: 31848948
-
Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
-
Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research.Plants (Basel). 2020 May 27;9(6):681. doi: 10.3390/plants9060681. Plants (Basel). 2020. PMID: 32471287 Free PMC article. Review.
-
Effect of Fusarium proliferatum infection on physiological, phytochemical, and nutrient responses in garlic.PeerJ. 2025 Jun 20;13:e19601. doi: 10.7717/peerj.19601. eCollection 2025. PeerJ. 2025. PMID: 40552038 Free PMC article.
-
Questioning ZnO, Ag, and Ag/ZnO nanoparticles as antimicrobial agents for textiles: Do they guarantee total protection against bacteria and SARS-CoV-2?J Photochem Photobiol B. 2022 Sep;234:112538. doi: 10.1016/j.jphotobiol.2022.112538. Epub 2022 Aug 4. J Photochem Photobiol B. 2022. PMID: 35964336 Free PMC article.
References
-
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002; 53: 1–11. - PubMed
-
- Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004; 166: 1213–1218.
-
- Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, http://www.ncbi.nlm.nih.gov/pubmed?term=Dizdaroglu%20M%5BAuthor%5D&cauth.... Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol. 2012; 46: 18–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

