mGlu1 tonically regulates levels of calcium-permeable AMPA receptors in cultured nucleus accumbens neurons through retinoic acid signaling and protein translation
- PMID: 30222904
- PMCID: PMC7556732
- DOI: 10.1111/ejn.14151
mGlu1 tonically regulates levels of calcium-permeable AMPA receptors in cultured nucleus accumbens neurons through retinoic acid signaling and protein translation
Abstract
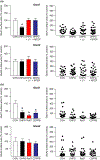
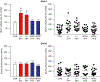
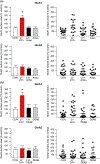
In several brain regions, ongoing metabotropic glutamate receptor 1 (mGlu1) transmission has been shown to tonically suppress synaptic levels of Ca2+ -permeable AMPA receptors (CP-AMPARs) while pharmacological activation of mGlu1 removes CP-AMPARs from these synapses. Consistent with this, we previously showed in nucleus accumbens (NAc) medium spiny neurons (MSNs) that reduced mGlu1 tone enables and mGlu1 positive allosteric modulation reverses the elevation of CP-AMPAR levels in the NAc that underlies enhanced cocaine craving in the "incubation of craving" rat model of addiction. To better understand mGlu1/CP-AMPAR interactions, we used a NAc/prefrontal cortex co-culture system in which NAc MSNs express high CP-AMPAR levels, providing an in vitro model for NAc MSNs after the incubation of cocaine craving. The non-specific group I orthosteric agonist dihydroxyphenylglycine (10 min) decreased cell surface GluA1 but not GluA2, indicating CP-AMPAR internalization. This was prevented by mGlu1 (LY367385) or mGlu5 (MTEP) blockade. However, a selective role for mGlu1 emerged in studies of long-term antagonist treatment. Thus, LY367385 (24 hr) increased surface GluA1 without affecting GluA2, whereas MTEP (24 hr) had no effect. In hippocampal neurons, scaling up of CP-AMPARs can occur through a mechanism requiring retinoic acid (RA) signaling and new GluA1 synthesis. Consistent with this, the LY367385-induced increase in surface GluA1 was blocked by anisomycin (translation inhibitor) or 4-(diethylamino)-benzaldehyde (RA synthesis inhibitor). Thus, mGlu1 transmission tonically suppresses cell surface CP-AMPAR levels, and decreasing mGlu1 tone increases surface CP-AMPARs via RA signaling and protein translation. These results identify a novel mechanism for homeostatic plasticity in NAc MSNs.
Keywords: GluA1; group I metabotropic glutamate receptors; homeostatic plasticity; primary culture; receptor trafficking.
© 2018 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Competing Interests
The authors have no conflicts of interest.
Figures

References
-
- Bellone C & Lüscher C (2005) mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci, 21, 1280–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DA038110/DA/NIDA NIH HHS/United States
- DA015835/DA/NIDA NIH HHS/United States
- K99 DA038110/DA/NIDA NIH HHS/United States
- DA038110/DA/NIDA NIH HHS/United States
- R01 DA009621/DA/NIDA NIH HHS/United States
- R01 DA015835/DA/NIDA NIH HHS/United States
- DA036950/DA/NIDA NIH HHS/United States
- DA009621/DA/NIDA NIH HHS/United States
- F31 DA036950/DA/NIDA NIH HHS/United States
- R37 DA015835/DA/NIDA NIH HHS/United States
- DA040414/DA/NIDA NIH HHS/United States
- F32 DA040414/DA/NIDA NIH HHS/United States
- F32 DA030844/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

