Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation
- PMID: 30223011
- PMCID: PMC6541932
- DOI: 10.1016/j.bbi.2018.09.018
Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation
Abstract
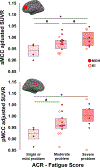
Fibromyalgia (FM) is a poorly understood chronic condition characterized by widespread musculoskeletal pain, fatigue, and cognitive difficulties. While mounting evidence suggests a role for neuroinflammation, no study has directly provided evidence of brain glial activation in FM. In this study, we conducted a Positron Emission Tomography (PET) study using [11C]PBR28, which binds to the translocator protein (TSPO), a protein upregulated in activated microglia and astrocytes. To enhance statistical power and generalizability, we combined datasets collected independently at two separate institutions (Massachusetts General Hospital [MGH] and Karolinska Institutet [KI]). In an attempt to disentangle the contributions of different glial cell types to FM, a smaller sample was scanned at KI with [11C]-L-deprenyl-D2 PET, thought to primarily reflect astrocytic (but not microglial) signal. Thirty-one FM patients and 27 healthy controls (HC) were examined using [11C]PBR28 PET. 11 FM patients and 11 HC were scanned using [11C]-L-deprenyl-D2 PET. Standardized uptake values normalized by occipital cortex signal (SUVR) and distribution volume (VT) were computed from the [11C]PBR28 data. [11C]-L-deprenyl-D2 was quantified using λ k3. PET imaging metrics were compared across groups, and when differing across groups, against clinical variables. Compared to HC, FM patients demonstrated widespread cortical elevations, and no decreases, in [11C]PBR28 VT and SUVR, most pronounced in the medial and lateral walls of the frontal and parietal lobes. No regions showed significant group differences in [11C]-L-deprenyl-D2 signal, including those demonstrating elevated [11C]PBR28 signal in patients (p's ≥ 0.53, uncorrected). The elevations in [11C]PBR28 VT and SUVR were correlated both spatially (i.e., were observed in overlapping regions) and, in several areas, also in terms of magnitude. In exploratory, uncorrected analyses, higher subjective ratings of fatigue in FM patients were associated with higher [11C]PBR28 SUVR in the anterior and posterior middle cingulate cortices (p's < 0.03). SUVR was not significantly associated with any other clinical variable. Our work provides the first in vivo evidence supporting a role for glial activation in FM pathophysiology. Given that the elevations in [11C]PBR28 signal were not also accompanied by increased [11C]-L-deprenyl-D2 signal, our data suggests that microglia, but not astrocytes, may be driving the TSPO elevation in these regions. Although [11C]-L-deprenyl-D2 signal was not found to be increased in FM patients, larger studies are needed to further assess the role of possible astrocytic contributions in FM. Overall, our data support glial modulation as a potential therapeutic strategy for FM.
Keywords: Astrocytes; Chronic overlapping pain conditions; Deprenyl-D2; Fibromyalgia; Functional pain; MRI/PET; Microglia; Neuroimmunology; Neuroinflammation; Positron emission tomography; TSPO.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors report no conflicts of interest
Figures
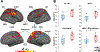

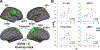

References
-
- Abourbeh G, Theze B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dolle F, Tavitian B, Boisgard R, 2012. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [(1)(8)F]DPA-714. J Neurosci 32, 5728–5736. - PMC - PubMed
-
- Albrecht DS, Normandin MD, Shcherbinin S, Wooten DW, Schwarz AJ, Zurcher NR, Barth VN, Guehl NJ, Johnson-Akeju O, Atassi N, Veronese M, Turkheimer F, Hooker JM, Loggia ML, 2017. Pseudo-reference regions for glial imaging with (11)C-PBR28: investigation in two clinical cohorts. J Nucl Med. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

