Lost medicines: a longer view of the pharmaceutical industry with the potential to reinvigorate discovery
- PMID: 30223039
- PMCID: PMC7245331
- DOI: 10.1016/j.drudis.2018.09.006
Lost medicines: a longer view of the pharmaceutical industry with the potential to reinvigorate discovery
Abstract
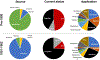
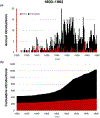
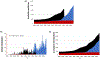
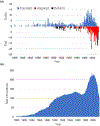
It is widely understood that the 1962 Kefauver-Harris Amendment to the Food, Drug and Cosmetics Act ushered in the modern regulation of medicines requiring a combination of safety and efficacy. However, fewer appreciate the amendment was applied retroactively to virtually all medicines sold in the USA. For various reasons, many medicines faded into history. Here, we identify and analyze >1600 medicines (including over-the-counter drugs) and their innovators prior to the enactment of Kefauver-Harris. We report 880 of these past medicines are no longer accessible. This project also reveals new insight into the pharmaceutical enterprise, which reveals an industry already mature and beginning to retract before enactment of the legislation. Beyond its historical implications, the recollection of these medicines could offer potential starting points for the future development of much-needed drugs.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The evolution of human medicines control from a national to an international perspective.Adverse Drug React Toxicol Rev. 1998 Mar;17(1):19-50. Adverse Drug React Toxicol Rev. 1998. PMID: 9638280 No abstract available.
-
Drug discovery: a historical perspective.Science. 2000 Mar 17;287(5460):1960-4. doi: 10.1126/science.287.5460.1960. Science. 2000. PMID: 10720314
-
Historical perspectives on the marketing of medicines.Publ Am Inst Hist Pharm. 1997;16:263-76. Publ Am Inst Hist Pharm. 1997. PMID: 11619898 No abstract available.
-
Unapproved drugs in the United States and the Food and Drug Administration.Adv Ther. 2011 Oct;28(10):842-56. doi: 10.1007/s12325-011-0059-4. Epub 2011 Sep 1. Adv Ther. 2011. PMID: 21894470 Review.
-
Data Integrity: History, Issues, and Remediation of Issues.PDA J Pharm Sci Technol. 2018 Mar-Apr;72(2):105-116. doi: 10.5731/pdajpst.2017.007765. Epub 2017 Nov 20. PDA J Pharm Sci Technol. 2018. PMID: 29158286 Review.
Cited by
-
Paving New Roads Towards Biodiversity-Based Drug Development in Brazil: Lessons from the Past and Future Perspectives.Rev Bras Farmacogn. 2021;31(5):505-518. doi: 10.1007/s43450-021-00181-2. Epub 2021 Sep 17. Rev Bras Farmacogn. 2021. PMID: 34548709 Free PMC article. Review.
References
-
- Goodrich WW (1963) FDA’s Regulation under the Kefauver-Harris Drug Amendments of 1962. Food Drug Cosmetics Law Journal 18, 561
-
- Kinch MS, ed. (2016) A Prescription for Change, Chapel Hill, NC: UNC Press
-
- Kinch MS et al. (2014) An overview of FDA-approved new molecular entities: 1827–2013. Drug Discov. Today 19, 1033–1039 - PubMed
-
- Munos B (2009) Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov 8, 959–968 - PubMed
-
- Scannell JW et al. (2012) Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov 11, 191–200 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

