Asymmetric Synthesis of (-)-6-Desmethyl-Fluvirucinine A₁ via Conformationally-Controlled Diastereoselective Lactam-Ring Expansions
- PMID: 30223428
- PMCID: PMC6225218
- DOI: 10.3390/molecules23092351
Asymmetric Synthesis of (-)-6-Desmethyl-Fluvirucinine A₁ via Conformationally-Controlled Diastereoselective Lactam-Ring Expansions
Abstract
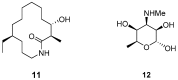
The versatile synthesis of (-)-6-desmethyl-fluvirucinine A₁ was accomplished at a 24% overall yield through a thirteen-step process from a known vinylpiperidine. The key part involved the elaboration of the distal stereocenters and a macrolactam skeleton via conformationally-induced diastereocontrol and the iterative aza-Claisen rearrangements of lactam precursors.
Keywords: amidoalkylation; aza-Claisen rearrangement; fluvirucinine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
First total synthesis and structural confirmation of fluvirucinine A2 via an iterative ring expansion strategy.Org Lett. 2010 May 7;12(9):2040-3. doi: 10.1021/ol100521v. Org Lett. 2010. PMID: 20345160
-
Highly efficient asymmetric synthesis of fluvirucinine A1 via Zr-catalyzed asymmetric carboalumination of alkenes (ZACA)-lipase-catalyzed acetylation tandem process.Org Lett. 2008 Jan 17;10(2):193-5. doi: 10.1021/ol702272d. Epub 2007 Dec 13. Org Lett. 2008. PMID: 18076181
-
A stereo-controlled access to functionalized macrolactams via an aza-Claisen rearrangement.Org Biomol Chem. 2012 Jan 21;10(3):561-8. doi: 10.1039/c1ob06733h. Epub 2011 Nov 22. Org Biomol Chem. 2012. PMID: 22108849
-
Recent progress towards transition metal-catalyzed synthesis of γ-lactams.Org Biomol Chem. 2014 Mar 28;12(12):1833-45. doi: 10.1039/c3ob42181c. Epub 2014 Jan 29. Org Biomol Chem. 2014. PMID: 24473105 Review.
-
Recent developments in the use of catalytic asymmetric ammonium enolates in chemical synthesis.Chem Rev. 2007 Dec;107(12):5596-605. doi: 10.1021/cr0683764. Chem Rev. 2007. PMID: 18072805 Review. No abstract available.
Cited by
-
Investigation of Grignard Reagent as an Advanced Base for Aza-Claisen Rearrangement.Molecules. 2019 Dec 16;24(24):4597. doi: 10.3390/molecules24244597. Molecules. 2019. PMID: 31888158 Free PMC article.
-
Stereogenic Centers.Molecules. 2018 Nov 13;23(11):2964. doi: 10.3390/molecules23112964. Molecules. 2018. PMID: 30428626 Free PMC article.
References
-
- Naruse N., Tenmyo O., Kawano K., Tomita K., Ohgusa N., Miyaki T., Konishi M., Oki T. Fluvirucins A1, A2, B1, B2, B3, B4 and B5, new antibiotics active against influenza A virus. I. Production, isolation, chemical properties and biological activities. J. Antibiot. 1991;44:733–740. doi: 10.7164/antibiotics.44.733. - DOI - PubMed
-
- Hegde V.R., Patel M.G., Gullo V.P., Ganguly A.K., Sarre O., Puar M.S., McPhail A.T. Macrolactams: A new class of antifungal agents. J. Am. Chem. Soc. 1990;112:6403–6405. doi: 10.1021/ja00173a042. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

