Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity
- PMID: 30223482
- PMCID: PMC6164259
- DOI: 10.3390/nu10091304
Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity
Abstract
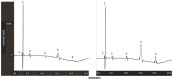
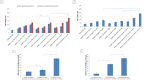
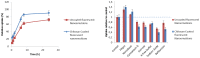
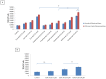
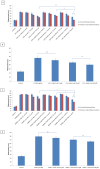
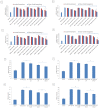
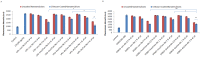
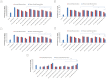
Doxorubicin is a highly active antineoplastic agent, but its clinical use is limited because of its cardiotoxicity. Although nutraceuticals endowed with anti-inflammatory properties exert cardioprotective activity, their bioavailability and stability are inconsistent. In an attempt to address this issue, we evaluated whether bioavailable nanoemulsions loaded with nutraceuticals (curcumin and fresh and dry tomato extracts rich in lycopene) protect cardiomyoblasts (H9C2 cells) from doxorubicin-induced toxicity. Nanoemulsions were produced with a high-pressure homogenizer. H9C2 cells were incubated with nanoemulsions loaded with different nutraceuticals alone or in combination with doxorubicin. Cell viability was evaluated with a modified MTT method. The levels of the lipid peroxidation products malondialdehyde (MDA) and 4-hydroxy-2-butanone (4-HNA), and of the cardiotoxic-related interleukins IL-6, IL-8, IL-1β and IL-10, tumor necrosis factor-alpha (TNF-α), and nitric oxide were analyzed in cardiomyoblasts. The hydrodynamic size of nanoemulsions was around 100 nm. Cell viability enhancement was 35⁻40% higher in cardiomyoblasts treated with nanoemulsion + doxorubicin than in cardiomyoblasts treated with doxorubicin alone. Nanoemulsions also protected against oxidative stress as witnessed by a reduction of MDA and 4-HNA. Notably, nanoemulsions inhibited the release of IL-6, IL-8, IL-1β, TNF-α and nitric oxide by around 35⁻40% and increased IL-10 production by 25⁻27% versus cells not treated with emulsions. Of the nutraceuticals evaluated, lycopene-rich nanoemulsions had the best cardioprotective profile. In conclusion, nanoemulsions loaded with the nutraceuticals described herein protect against cardiotoxicity, by reducing inflammation and lipid oxidative stress. These results set the stage for studies in preclinical models.
Keywords: cardiology; cytokines; doxorubicin; nanomedicine; nutraceuticals.
Conflict of interest statement
The authors declare no conflict of interest. Any role of the funding sponsors in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Maurea N., Coppola C., Piscopo G., Galletta F., Riccio G., Esposito E., De Lorenzo C., De Laurentiis M., Spallarossa P., Mercuro G. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J. Cardiovasc. Med. 2016;17(Suppl. 1):e19–e26. doi: 10.2459/JCM.0000000000000377. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

