Can the Fact That Myelin Proteins Are Old and Break down Explain the Origin of Multiple Sclerosis in Some People?
- PMID: 30223497
- PMCID: PMC6162792
- DOI: 10.3390/jcm7090281
Can the Fact That Myelin Proteins Are Old and Break down Explain the Origin of Multiple Sclerosis in Some People?
Abstract
Recent discoveries may change the way that multiple sclerosis (MS) is viewed, particularly with regard to the reasons for the untoward immune response. The fact that myelin proteins are long-lived, and that by the time we are adults, they are extensively degraded, alters our perspective on the reasons for the onset of autoimmunity and the origin of MS. For example, myelin basic protein (MBP) from every human brain past the age of 20 years, is so greatly modified, that it is effectively a different protein from the one that was laid down in childhood. Since only a subset of people with such degraded MBP develop MS, a focus on understanding the mechanism of immune responses to central nervous system (CNS) antigens and cerebral immune tolerance appear to be worthwhile avenues to explore. In accord with this, it will be productive to examine why all people, whose brains contain large quantities of a "foreign antigen", do not develop MS. Importantly for the potential causation of MS, MBP from MS patients breaks down differently from the MBP in aged controls. If the novel structures formed in these MS-specific regions are particularly antigenic, it could help explain the origin of MS. If verified, these findings could provide an avenue for the rational synthesis of drugs to prevent and treat MS.
Keywords: age-related; isoaspartate; myelin basic protein; posttranslational modification; protein decomposition; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



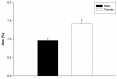
 ) and female (
) and female ( ) subjects. Elevated levels of
) subjects. Elevated levels of References
-
- Patsopoulos N., Baranzini S.E., Santaniello A., Shoostari P., Cotsapas C., Wong G., Beecham A.H., James T., Replogle J., Vlachos I., et al. The multiple sclerosis genomic map: Role of peripheral immune cells and resident microglia in susceptibility. bioRxiv. 2017;1:143933.
-
- Rothhammer V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C.C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

