Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding?
- PMID: 30223513
- PMCID: PMC6164003
- DOI: 10.3390/biom8030094
Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding?
Abstract
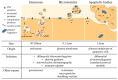
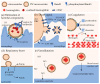
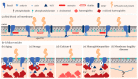
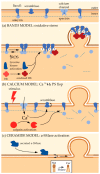
Extracellular vesicles (EVs) contribute to several pathophysiological processes and appear as emerging targets for disease diagnosis and therapy. However, successful translation from bench to bedside requires deeper understanding of EVs, in particular their diversity, composition, biogenesis and shedding mechanisms. In this review, we focus on plasma membrane-derived microvesicles (MVs), far less appreciated than exosomes. We integrate documented mechanisms involved in MV biogenesis and shedding, focusing on the red blood cell as a model. We then provide a perspective for the relevance of plasma membrane lipid composition and biophysical properties in microvesiculation on red blood cells but also platelets, immune and nervous cells as well as tumor cells. Although only a few data are available in this respect, most of them appear to converge to the idea that modulation of plasma membrane lipid content, transversal asymmetry and lateral heterogeneity in lipid domains may play a significant role in the vesiculation process. We suggest that lipid domains may represent platforms for inclusion/exclusion of membrane lipids and proteins into MVs and that MVs could originate from distinct domains during physiological processes and disease evolution.
Keywords: calcium; ceramide; cholesterol; cytoskeleton; lipid domains; microvesicle; oxidative stress; raft; red blood cell; sphingomyelinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

