PCB11 Metabolite, 3,3'-Dichlorobiphenyl-4-ol, Exposure Alters the Expression of Genes Governing Fatty Acid Metabolism in the Absence of Functional Sirtuin 3: Examining the Contribution of MnSOD
- PMID: 30223548
- PMCID: PMC6162768
- DOI: 10.3390/antiox7090121
PCB11 Metabolite, 3,3'-Dichlorobiphenyl-4-ol, Exposure Alters the Expression of Genes Governing Fatty Acid Metabolism in the Absence of Functional Sirtuin 3: Examining the Contribution of MnSOD
Abstract
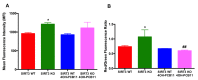
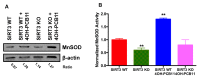
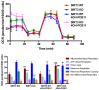
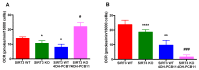
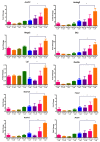
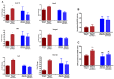
Although the production of polychlorinated biphenyls (PCBs) is prohibited, the inadvertent production of certain lower-chlorinated PCB congeners still threatens human health. We and others have identified 3,3'-dichlorobiphenyl (PCB11) and its metabolite, 3,3'-dichlorobiphenyl-4-ol (4OH-PCB11), in human blood, and there is a correlation between exposure to this metabolite and mitochondrial oxidative stress in mammalian cells. Here, we evaluated the downstream effects of 4OH-PCB11 on mitochondrial metabolism and function in the presence and absence of functional Sirtuin 3 (SIRT3), a mitochondrial fidelity protein that protects redox homeostasis. A 24 h exposure to 3 μM 4OH-PCB11 significantly decreased the cellular growth and mitochondrial membrane potential of SIRT3-knockout mouse embryonic fibroblasts (MEFs). Only wild-type cells demonstrated an increase in Manganese superoxide dismutase (MnSOD) activity in response to 4OH-PCB11⁻induced oxidative injury. This suggests the presence of a SIRT3-mediated post-translational modification to MnSOD, which was impaired in SIRT3-knockout MEFs, which counters the PCB insult. We found that 4OH-PCB11 increased mitochondrial respiration and endogenous fatty-acid oxidation-associated oxygen consumption in SIRT3-knockout MEFs; this appeared to occur because the cells exhausted their reserve respiratory capacity. To determine whether these changes in mitochondrial respiration were accompanied by similar changes in the regulation of fatty acid metabolism, we performed quantitative real-time polymerase chain reaction (qRT-PCR) after a 24 h treatment with 4OH-PCB11. In SIRT3-knockout MEFs, 4OH-PCB11 significantly increased the expression of ten genes controlling fatty acid biosynthesis, metabolism, and transport. When we overexpressed MnSOD in these cells, the expression of six of these genes returned to the baseline level, suggesting that the protective role of SIRT3 against 4OH-PCB11 is partially governed by MnSOD activity.
Keywords: 4OH-PCB11; MnSOD; PCB 11; Sirtuin 3; fatty acid metabolism; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

