Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering
- PMID: 30223550
- PMCID: PMC6165032
- DOI: 10.3390/jfb9030053
Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering
Abstract
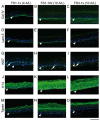
There is a high incidence of failure and recurrence for chronic skin wounds following conventional therapies. To promote healing, the use of skin substitutes containing living cells as wound dressings has been proposed. The aim of this study was to produce a scaffold-free cell-based bilayered tissue-engineered skin substitute (TES) containing living fibroblasts and keratinocytes suitable for use as wound dressing, while considering production time, handling effort during the manufacturing process, and stability of the final product. The self-assembly method, which relies on the ability of mesenchymal cells to secrete and organize connective tissue sheet sustaining keratinocyte growth, was used to produce TESs. Three fibroblast-seeding densities were tested to produce tissue sheets. At day 17, keratinocytes were added onto 1 or 3 (reference method) stacked tissue sheets. Four days later, TESs were subjected either to 4, 10, or 17 days of culture at the air⁻liquid interface (A/L). All resulting TESs were comparable in terms of their histological aspect, protein expression profile and contractile behavior in vitro. However, signs of extracellular matrix (ECM) digestion that progressed over culture time were noted in TESs produced with only one fibroblast-derived tissue sheet. With lower fibroblast density, the ECM of TESs was almost completely digested after 10 days A/L and lost histological integrity after grafting in athymic mice. Increasing the fibroblast seeding density 5 to 10 times solved this problem. We conclude that the proposed method allows for a 25-day production of a living TES, which retains its histological characteristics in vitro for at least two weeks.
Keywords: bilayered skin substitutes; culture techniques; regenerative medicine; skin equivalent; skin ulcer; tissue culture; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bosanquet N., Franks P., Moffatt C., Connolly M., Oldroyd M., Brown P., Greenhalgh R., McCollum C. Community leg ulcer clinics: Cost-effectiveness. Health Trends. 1993;25:146–148. - PubMed
-
- Nelson E.A., Adderley U. Venous leg ulcers. [(accessed on 15 January 2016)];2016 Available online: https://www.ncbi.nlm.nih.gov/pubmed/26771825.
-
- Ashby R.L., Gabe R., Ali S., Adderley U., Bland J.M., Cullum N.A., Dumville J.C., Iglesias C.P., Kang’ombe A.R., Soares M.O., et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (venous leg ulcer study iv, venus iv): A randomised controlled trial. Lancet. 2014;383:871–879. doi: 10.1016/S0140-6736(13)62368-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

